Abstract
Purpose
Total hip arthroplasty (THA) is a successful surgery for the treatment of hip osteoarthritis; however, the risk of a post-operative prosthetic joint infection (PJI) remains at 1% to 2%. The purpose of this study was to investigate the safety profile of using vancomycin powder (VP) to reduce infection rates by reviewing acute postoperative complications.
Materials and Methods
A retrospective review of 265 consecutive patients undergoing THA was performed. The first 128 patients, the control group, did not receive VP, and the subsequent 137 patients, the VP group, received VP at the time of wound closure. Patient demographic data, medical comorbidities, and perioperative information were compared.
Results
The primary outcome was a post-operative surgical complication within 90 days from surgery. The control and VP group's demographic, medical comorbidities and perioperative information data were statistically similar. Deep infection rate in the control group was 5.5%, whereas the deep infection rate in the VP group was 0.7% (P=0.031). Sterile wound complication rate was 4.4% in the VP group, and 0% in the control group (P=0.030). Remaining complications were not statistically different between the groups.
Conclusion
VP was associated with an increase rate of sterile wound complications compared to the control group. The rate of PJI was decreased with the use of VP. We do not recommend for or against the use of VP at time of wound closure to prevent PJI, and higher powered studies will need to be performed to demonstrate the efficacy of VP.
Total hip arthroplasty (THA) is a highly successful approach for the treatment of hip osteoarthritis, with a 92% to 94% survivorship at 7 to 12 year follow up 12345). Despite the overall success of THA, periprosthetic joint infection (PJI)-the most common cause of readmissions after total joint arthroplasty-occurs in approximately 1% to 2% of patients 16789). PJIs are associated with significant patient morbidity and a large financial burden on the healthcare system; current estimates suggest that healthcare costs for the treatment of PJI in the United States will exceed 1.62 billion US dollars by the year 202010).
Early postoperative PJI are likely caused by endogenous skin flora or exogenous bacteria that contaminate the joint space at the time of surgery1). The most common causative organism in early infections is Staphylococcus aureus, and both methicillin-resistant (MRSA) and methicillin-sensitive (MSSA) species are frequently cultured11). Equipped with the understanding that early infections occur from perioperative contamination, surgeons have developed well-established modalities to mitigate infection risks (e.g., prophylactic antibiotics, preoperative skin prep, sterile draping, antibiotic cement, and medical optimization prior to surgery)12). Despite the variety of preventative measures available, however, the infection rate during THA remains approximately 1–2%.
While the use of prophylactic systemic antibiotics is standard of care during joint arthroplasty and drastically reduce the risk of PJI, postoperative wounds have areas not reachable by intravenously delivered antibiotics (e.g., ischemic tissue or hematoma)1314). Adjunctive intrawound application of an antibiotic, however, may create a potent bactericidal environment by producing a high local tissue concentration of the antibiotic without relying on tissue blood supply15). In a mouse model, an intra-articular knee implant was surgically placed and bacterial colony counts were compared between a group who received intrawound vancomycin powder (VP) at the time of skin closure and a control group who received no VP. Bacterial colony counts were significantly decreased at the distal femur, joint capsule, and the implanted device in the VP group16). VP has been used by spine surgeons for a number of years and there is evidence demonstrating a reduction in surgical site infections. More recently, the use of VP has been gaining popularity among arthroplasty surgeons. Otte et al.17) assessed the use of intrawound VP in revision total knee arthroplasty (TKA) and revision THA, and found the infection rates in patients receiving intrawound VP was significantly lower than in patients who received no intrawound VP, 0.0% vs. 3.89%, respectively.
In a recent systematic review of VP in spinal surgery, Ghobrial et al.18) analyzed 16 cohort studies and found that the risk of SSI in patients who received intrawound VP vs. those that did not receive intrawound VP were 1.36% and 7.47%, respectively. While vancomycin is associated with adverse events (e.g., acute kidney injury, otoxicity, and anaphylaxis), this systematic review demonstrated a lower adverse event rate related to the VP of 0.3%. Our hypothesis is that VP is a safe and effective adjunct that can potentially decrease the rate of PJIs following THA.
After institutional review board approval, we retrospectively reviewed a series of 265 consecutive patients who underwent primary THA between June 2013 and February 2016 with a minimum of 3 months follow up. This cohort included all patients operated on by a single surgeon (corresponding author) in the given time period, and other than the adjuvant intrawound administration of VP at time of wound closure (starting in January 2015), the surgeon's perioperative management of patients was identical. In total, 128 patients received no VP during THA (all treated between June 2013 and prior to January 2015; control group) and 137 patients received VP during THA (treated between January 2015 and February 2016; VP group). Demographic information (e.g., age at surgery, sex, body mass index [BMI], and comorbidities) was collected. Comorbidities included hypertension, diabetes mellitus (DM), rheumatoid arthritis (RA), chronic kidney disease, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease (COPD), and American Society of Anesthesiologists (ASA) physical status scores.
All THA were performed using cementless implants and an anterior-based muscle sparing surgical approach to the hip19). The indication for surgery was debilitating hip pain with loss of function secondary to hip arthritis that did not respond to conservative treatment. All patients underwent a standard infection-prevention protocol including: i) perioperative intravenous (IV) administration of cefazolin (2 g) starting prior to skin incision and ending 24 hours after the end of the operation; ii) preoperative showering with chlorhexidine gluconate (the evening before surgery); iii) standard skin preparation with 2% chlorhexidine gluconate and 70% isopropyl alcohol (ChloraPrep; Mediflex, Leawood, KS, USA); and iv) wound closure with monofilament sutures. If a patient had a severe cephalosporin or penicillin allergy or history of MRSA, 1 g of IV vancomycin or 600 mg of IV clindamycin was administered prior to surgical incision in place of cefazolin. In the VP group, 1 g of VP was applied both intrapcapsularly and extracapsularly into the wound after final wound irrigation.
Postoperative complications (i.e., deep PJI, superficial infection and wound complications) were collected if diagnosed within 90 days of the initial surgery. Deep PJIs were those that met the periprosthetic infection criteria described by the International Consensus Meeting on Periprosthetic Joint Infections20). Superficial infections were surgical site infections that resolved with oral antibiotics and which did not require an additional operation. Wound complications were wound breakdowns that did not meet the aforementioned criteria for periprosthetic infection, and required a return to the operating room (OR) for wound debridement and closure. Intraoperative wound cultures were obtained following the onset of a wound complication, and were negative for infection. Perioperative medical complications (i.e., acute renal failure [ARF]-defined as an increase in serum creatinine of greater than 0.3 mg/dL, ototoxicity, aseptic loosening, hip dislocation, periprosthetic fracture, THA revision, return to OR, spacer, hip washout, death, myocardial infection [MI], stroke, deep vein thrombosis, pulmonary edema [PE] and anaphylaxis) were also quantified to evaluate the safety of VP.
Baseline demographics are presented in Table 1. Both groups were approximately 50% male with an average age of approximately 61 years and average BMI of 30 kg/m2. There were no significant differences between the groups with regards to age (P=0.837), sex (P=0.678), or BMI (P=0.851). The control group had a significantly longer (P<0.001) average follow-up time of 14.4 months (range, 3.0 to 34.9 months) compared with the VP group with an average follow-up of 8.2 months (range, 3.0 to 20.1 months).
Continuous data are presented using the mean, standard deviation and range and compared between the two groups using a t-test. Normality was assessed using the Kolmogorov-Smirnov test and normality plots. Categorical data are presented using counts and percentages for non-missing data. Groups were compared using the chi-square or Fisher's exact tests (expected cell counts <5). Odds ratios with 95% confidence intervals are presented where possible (non-zero cells) to compare complication rates between the two groups. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses and a P-value <0.05 was considered statistically significant.
ASA scores and percentage of smokers were not significantly different between the two groups. The control group had a significantly greater (P=0.046) number of patients with COPD (8.6%) compared to the VP group (2.9%). All other medical comorbidities were similar between the two groups (Table 2). The indication for THA was not statistically different between the groups (P=0.699), with 8 cases (6.2%) vs. 5 cases (3.7%) of avascular necrosis, 1 case (0.8%) vs. 1 case (0.7%) of inflammatory arthropathy and 119 cases (93.0%) vs. 131 cases (95.6%) of osteoarthritis in the control and VP groups, respectively.
The 115 patients (89.8%) of the control group vs. 120 patients (87.6%) of the VP group received IV cefazolin as prophylactic antibiotic. Of the remaining patients in the control group, 7 patients (5.5%) received IV vancomycin and 7 patients received (5.5%) IV clindamycin. Likewise, 13 patients (9.5%) of the VP group received IV vancomycin while the remaining 2 patients (1.5%) of the VP group received IV clindamycin. When comparing the groups, there were no significant differences in the use of prophylactic cefazolin (P=0.563), vancomycin (P=0.216), or clindamycin (P=0.094).
When comparing the rates complications between the two groups (Table 3), the control group had a higher rate of superficial infection and deep infection, and the VP group had a higher rate of wound complications. While the difference in prevalence of superficial infection was not significantly different (P=0.611), the difference in prevalence of deep PJI was statistically significant (P=0.031). Likewise, the higher rate of wound complications in the VP group was statistically significant (P=0.030) when compared to the control group.
Regarding the 7 patients (5.5%) in the control group with PJI (Table 4), all were managed by the infectious disease team and treated initially with a head and liner exchange and IV antibiotics. In those requiring a second procedure, the average time from index THA was 33 days. Of the 7 patients treated initially with head and liner exchange, infections cleared in 6 (no additional procedures were required), and the infection failed to clear in 1 (explant of all components and antibiotic spacer placement was required prior to re-implantation of a prosthetic hip).
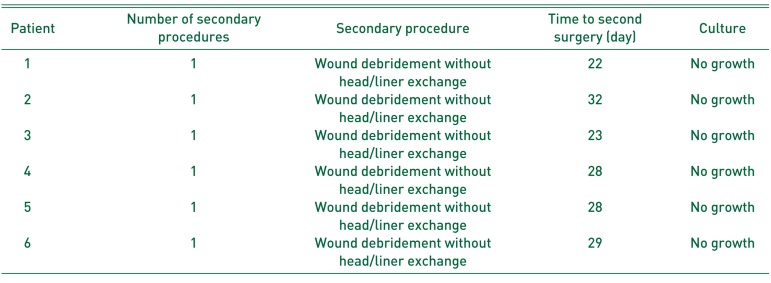
In the VP group, 1 patient (0.7%) with PJI underwent head and liner exchange 28 days after index THA (Table 5). The infection cleared in this patient and no additional procedures were required. The VP group also had 6 patients (4.4%) with wound complications. These wound complications did not meet criteria for PJI and were thought to be secondary to a seroma. These patients were treated with wound debridement and closure without head or liner exchange and no postoperative antibiotics. Intra-operative cultures were negative for bacterial growth for all 6 patients. The average time to second procedure from index THA was 27 days. The wounds of all 6 patients healed after the second operation and subsequent procedures were not required.
Staphylococcus was the most commonly isolated bacteria species and there 4, 2, and 1 cases of MSSA, MRSA and coagulase negative staphylococcus, respectively. There was also 1 case with no bacterial growth.
With regards to adverse events related to VP, no patients in the VP group developed ARF while 3 patients (2.3%) in the control group developed ARF which resolved by day of discharge (P=0.111). No patients in either group developed ototoxicity, aseptic loosening, death, MI, stroke, PE, or anaphylaxis.
VP has been well studied in the spine literature, and has been shown to reduce deep surgical site infections (SSIs) following the use of spinal instrumentation1521). Recently, a study was published demonstrating a reduced prevalence of PJI following revision TKA or THA using VP17). However, the safety and effectiveness of VP during THA has not been well established. One concern, specific to arthroplasty, is increased third body wear rates with the addition of VP. A mechanical in-vitro study attempted to address this question, and did not demonstrate increased third body polyethylene wear rates following cyclic loading with the addition of intra-articular VP22).
In our study, we observed a statistically significant lower rate of PJI in the VP group. The control group underwent the same standard preoperative infection prevention protocol and did not have an increased rate of comorbidities associated increased rates of PJI (i.e., DM or RA) compared with the VP group. The control group did, however, have a statistically significant increased rate of COPD-a comorbidity which is associated with increased rates of PJI-and this could be a confounding variable for the increased PJI rate in the control group23). While the statistically significant decreased rate of PJI in the VP group may be explained by VP use, it is unclear why our control group PJI rate of 5.4% is much higher than the 1% to 2% PJI rate reported in the literature16789), and this is a major limitation of this study. While the cause is unclear, during this period the hospital had an increase in infection rates and a thorough evaluation by infectious disease experts was performed with no clear cause identified. One consideration is that 6 of the 7 patients with PJI had a BMI greater than 30 kg/m2 which has been shown to be a risk factor for PJI in patients having THA through an anterior approach2425). As mentioned, the higher rate of COPD in the control group could also have contributed to a higher infection rate23). Lastly, one of the PJI in the control group received clindamycin for pre-operative prophylaxis, a bacteriostatic antibiotic, which could be associated with higher rates of PJI. Despite this limitation, the decreased prevalence of infections in the VP group does agree with the findings of multiple studies that have evaluated the efficacy of VP in reducing postoperative infections.
The use of topical vancomycin was first reported in 1989 when the application of topical vancomycin to the sternum in cardiothoracic patients reduced rates of sternal infection from 3.6% to 0.45%26). Similarly, multiple studies have shown that vancomycin decreases the rate of postoperative infections in patients undergoing spinal surgery. The first large retrospective study investigating the clinical efficacy of VP was published in 2011 and reviewed 1,732 consecutive spinal fusions and showed a reduction in infection rate from 2.6% to 0.2%27). Furthermore, O'Neil et al.28) found that the use of intrawound VP in patients undergoing posterior spinal stabilization after spine trauma reduced infection rates from 13% to 0%. Likewise, the use of VP in posterior cervical spine fusion has been shown to be effective with a decrease in infection rates from 10.9% to 2.5%29). However, in a prospective randomized controlled trial comparing 433 patients receiving VP to a control group of 474 patients receiving no VP, Tubaki et al.30) found no statistical difference in infection rates (1.6% in both groups). The authors hypothesized that the addition of VP may not be effective when the incidence of postoperative infection is low. Similarly, VP in THA may be an efficacious adjunct in reducing infection rates when the incidence of PJI is high as observed in our study.
Our study also shows that intrawound VP in THA is safe with no cases of nephrotoxicity, ototoxicity or anaphylaxis. This finding agrees with the safety profile of intrawound VP in the spine literature. A number of studies have investigated serum concentration levels of vancomycin using local VP, and all report serum concentrations well below toxic levels23272829). In a recent systematic review investigating the complications associated with the intrawound use of VP in 6,701 cases of spine surgery, the authors found one reported case of nephrotoxicity, one case of ototoxicity, and 19 cases of seroma formation18).
Similar to reports in the spine literature, we note a statistically significant increase in sterile seroma formation requiring operative management in patients receiving VP15). Despite this known association, the mechanism of the seroma formation remains unclear. One possibility is that a local inflammatory reaction produced by the VP itself or an inflammatory reaction produced by the body's response to the foreign VP is responsible. Seroma formation following THA, although not as deleterious as a PJI, still requires reoperation and needs to be considered prior to VP use.
The adjunctive use of VP during wound closure is a trending modality to reduce rates of PJI. Here we evaluated the impact of adjunctive intrawound use of VP during THA on the rate of infections and observed an increase in the overall risk of sterile wound complications with the use of VP but a decreased rate of PJI. This study involved a relatively small sample size; however, and a second, higher-powered study should be performed to validate the results. Based on the results of this study, we cannot recommend for or against the use of VP during THA, however one needs to be aware of the potential benefits vs risks of adjunctive intrawound use of VP.
References
1. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016; 387:386–394. PMID: 26135702.

2. Garcia-Cimbrelo E, Cruz-Pardos A, Madero R, Ortega-Andreu M. Total hip arthroplasty with use of the cementless Zweymüller Alloclassic system. A ten to thirteen-year follow-up study. J Bone Joint Surg Am. 2003; 85-A:296–303. PMID: 12571308.
3. D'Angelo F, Murena L, Vulcano E, Zatti G, Cherubino P. Seven to twelve year results with Versys ET cementless stem. A retrospective study of 225 cases. Hip Int. 2010; 20:81–86. PMID: 20235069.
4. Castoldi F, Rossi R, La Russa M, Sibelli P, Rossi P, Ranawat AS. Ten-year survivorship of the Anatomique Benoist Girard I total hip arthroplasty. J Arthroplasty. 2007; 22:363–368. PMID: 17400092.

5. Herrera A, Canales V, Anderson J, García-Araujo C, Murcia-Mazón A, Tonino AJ. Seven to 10 years followup of an anatomic hip prosthesis: an international study. Clin Orthop Relat Res. 2004; (423):129–137.
6. Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty. Acta Orthop. 2009; 80:639–645. PMID: 19995313.

7. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008; 23:984–991. PMID: 18534466.

8. Zmistowski B, Restrepo C, Hess J, Adibi D, Cangoz S, Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Joint Surg Am. 2013; 95:1869–1876. PMID: 24132361.
9. Ilchmann T, Zimmerli W, Bolliger L, Graber P, Clauss M. Risk of infection in primary, elective total hip arthroplasty with direct anterior approach or lateral transgluteal approach: a prospective cohort study of 1104 hips. BMC Musculoskelet Disord. 2016; 17:471. PMID: 27842584.

10. Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012; 27(8 Suppl):61–65.e1. PMID: 22554729.

11. Parvizi J, Heller S, Berend KR, Della Valle CJ, Springer BD. Periprosthetic joint infection: the algorithmic approach and emerging evidence. Instr Course Lect. 2015; 64:51–60. PMID: 25745894.
12. Shahi A, Parvizi J. Prevention of periprosthetic joint infection. Arch Bone Jt Surg. 2015; 3:72–81. PMID: 26110171.
13. AlBuhairan B, Hind D, Hutchinson A. Antibiotic prophylaxis for wound infections in total joint arthroplasty: a systematic review. J Bone Joint Surg Br. 2008; 90:915–919. PMID: 18591602.
14. Hanssen AD, Osmon DR, Patel R. Local antibiotic delivery systems: where are we and where are we going? Clin Orthop Relat Res. 2005; (437):111–114.
15. Kang DG, Holekamp TF, Wagner SC, Lehman RA Jr. Intrasite vancomycin powder for the prevention of surgical site infection in spine surgery: a systematic literature review. Spine J. 2015; 15:762–770. PMID: 25637469.

16. Edelstein AI, Weiner JA, Cook RW, et al. Intra-articular vancomycin powder eliminates methicillin-resistant S. aureus in a rat model of a contaminated intra-articular implant. J Bone Joint Surg Am. 2017; 99:232–238. PMID: 28145954.

17. Otte JE, Politi JR, Chambers B, Smith CA. Intrawound vancomycin powder reduces early prosthetic joint infections in revision hip and knee arthroplasty. Surg Technol Int. 2017; 30:284–289. PMID: 28182821.
18. Ghobrial GM, Cadotte DW, Williams K Jr, Fehlings MG, Harrop JS. Complications from the use of intrawound vancomycin in lumbar spinal surgery: a systematic review. Neurosurg Focus. 2015; 39:E11.

19. Hansen BJ, Hallows RK, Kelley SS. The Rottinger approach for total hip arthroplasty: technique and review of the literature. Curr Rev Musculoskelet Med. 2011; 4:132–138. PMID: 21826433.

20. Parvizi J, Gehrke T, Chen AF. Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J. 2013; 95-B:1450–1452. PMID: 24151261.

21. Bakhsheshian J, Dahdaleh NS, Lam SK, Savage JW, Smith ZA. The use of vancomycin powder in modern spine surgery: systematic review and meta-analysis of the clinical evidence. World Neurosurg. 2015; 83:816–823. PMID: 25535069.

22. Qadir R, Ochsner JL, Chimento GF, Meyer MS, Waddell B, Zavatsky JM. Establishing a role for vancomycin powder application for prosthetic joint infection prevention-results of a wear simulation study. J Arthroplasty. 2014; 29:1449–1456. PMID: 24636904.

23. Everhart JS, Andridge RR, Scharschmidt TJ, Mayerson JL, Glassman AH, Lemeshow S. Development and validation of a preoperative surgical site infection risk score for primary or revision knee and hip arthroplasty. J Bone Joint Surg Am. 2016; 98:1522–1532. PMID: 27655979.

24. Jahng KH, Bas MA, Rodriguez JA, Cooper HJ. Risk factors for wound complications after direct anterior approach hip arthroplasty. J Arthroplasty. 2016; 31:2583–2587. PMID: 27267230.

25. Purcell RL, Parks NL, Gargiulo JM, Hamilton WG. Severely obese patients have a higher risk of infection after direct anterior approach total hip arthroplasty. J Arthroplasty. 2016; 31(9 Suppl):162–165.

26. Vander Salm TJ, Okike ON, Pasque MK, et al. Reduction of sternal infection by application of topical vancomycin. J Thorac Cardiovasc Surg. 1989; 98:618–622. PMID: 2796369.

27. Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: efficacy, drug levels, and patient outcomes. Spine (Phila Pa 1976). 2011; 36:2084–2088. PMID: 21304438.
28. O'Neill KR, Smith JG, Abtahi AM, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J. 2011; 11:641–646. PMID: 21600853.
29. Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 2013; 38:991–994. PMID: 23324930.

30. Tubaki VR, Rajasekaran S, Shetty AP. Effects of using intravenous antibiotic only versus local intrawound vancomycin antibiotic powder application in addition to intravenous antibiotics on postoperative infection in spine surgery in 907 patients. Spine (Phila Pa 1976). 2013; 38:2149–2155. PMID: 24048091.

Table 1
Patient Demographics for Each Group

Table 2
Comorbidities in Each Group
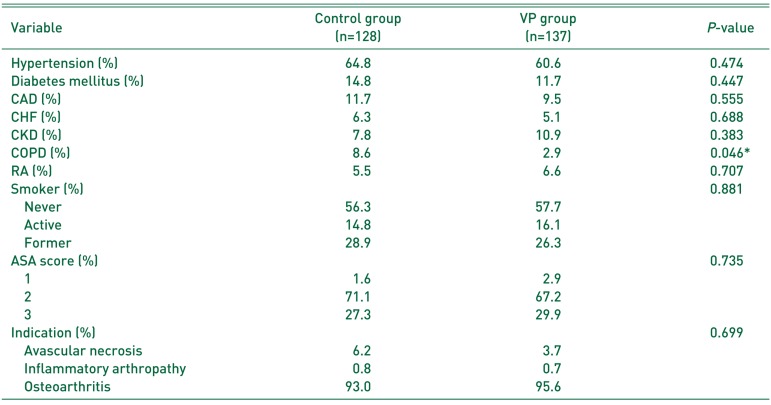
Table 3
Comparison of Complications between Groups
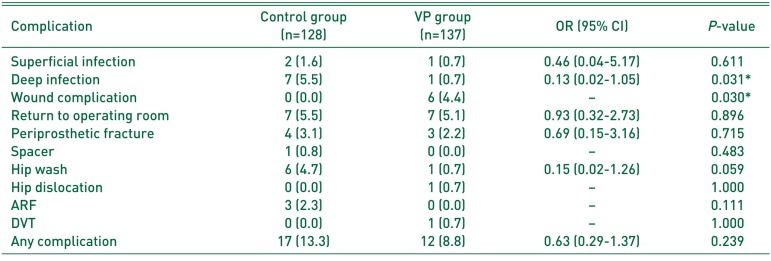
Table 4
Description of Prosthetic Joint Infection Patients in Each Group
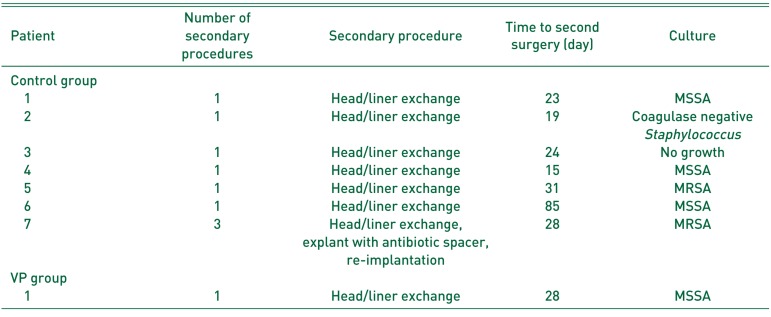
Table 5
Description of Vancomycin Powder Group Wound Complication Patient




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download