Abstract
Purpose
We evaluated the medium- to long-term outcomes of cortical strut allografts used to treat periprosthetic bone defects to better understand the correlation between radiological and clinical outcomes.
Materials and Methods
We retrospectively reviewed outcomes from 19 patients undergoing cortical strut allografts to treat periproshtetic bone defects from 2001 to 2015. The mean age at index operation was 59.4 years and the average follow-up period was 8.6 years. Surgeries were performed because of aseptic loosening (n=9), periprosthetic fractures (n=5), and infections (n=5). Each case was characterized and described in detail including the length of allograft and the union period; possible correlations between allograft length and detailed classification and union period was analyzed. Clinical evaluations included the Harris hip score and Kaplan-Meier survivorship.
Results
In revision total hip arthroplasty (THA), the average length of allografts used in patients experiencing fractures was significantly longer than those with aseptic loosening or infection. Of the 19 cases, incorporation was observed in 18 cases (94.7%). The average time to incorporation was 21.2 months and the time to incorporation was not significantly different among the two groups (fracture vs. aseptic loosening or infection). No positive correlation was identified between the length of allograft and incorporation period or in the time to cortical strut allograft incorporation among Paprosky or Vancouver subgroups.
As the incidence of total hip arthroplasties (THA) increases, the need for revision THA or open reduction and internal fixation (ORIF) for periprosthetic fractures also grows12). When attempting revision THA in patients with severe bone loss or in cases where firm fixation with the femoral stem at periprosthetic fracture is challenging34), cortical strut allografting may improve structural support. A number of cortical strut allografting applications exist (e.g., large bone defects like a cortical autograft and spine surgery to withstand compressive force). Studies have shown that the strength and speed of fixation greatly impacts a patient's ability to perform weight-bearing exercises.
While studies have shown that the success rate of incorporation and reconstruction of cortical strut allograft is between 80% to 100%56), there are relatively few studies reporting on the medium- to long-term outcomes of cortical strut allografts. The purpose of this study is to: i) evaluate outcomes related to the grafted bone using medium- to long-term radiological follow-ups of cortical strut allografts used to treat periprosthetic bone defects and ii) assess possible clinical correlations in revision THA and ORIF.
A retrospective study was carried out with 29 patients who, between December 2001 and December 2015, underwent a revision THA with cortical strut allografting due to aseptic loosening or infection, and an augmentation of cortical strut allograft during ORIF for periprosthetic fracture. In case of infection THA, two stage operation was performed. Radiological and clinical outcomes were available in 19 cases (19 patients); ten patients were excluded because of follow-up loss (n=2), death during the follow-up period (n=4) and follow up of less than 2 years (n=4). The study protocol was approved by Institutional Review Boards of Inha University Hospital (INHAUH 2017-03-025).
As shown in Table 1, the mean age of the 19 patients (10 male and 9 female) at index operation was 59.4±12.1 years (range, 43–84 years). The average period from primary surgery to revision surgery was 8.3±6.7 years (7 days–21.7 years). The average follow-up period was 8.6±3.6 years (range, 2.3–13.8 years). Revision THA were conducted because of aseptic loosening of THA implant (n=9; 47.4%), periprosthetic fractures (n=5; 26.3%), and infection (n=5; 26.3%). All surgeries were performed by a single surgeon (corresponding author).
The cases of revision THA due to aseptic loosening or infection were further classified using the Paprosky system7), and cases requiring ORIF because of fractures using the Vancouver system8). The length of cortical strut allograft was measured radiologically and we compared the lengths of cortical strut allografts used for aseptic loosening of THA or infection vs. the length of cortical strut allografts used for periprosthetic fractures. Incorporation was characterized by evaluating the time when bridging was observed through the whole contact area between the grafted bone and the host bone during the follow-up period.
The study compared the time to incorporation of the cortical strut allografts in aseptic loosening or infection of THA and the period in periprosthetic fractures, and evaluated whether the length of the cortical strut allograft correlated with the time to incorporation. Additionally, the duration of incorporation of the cortical strut allograft among Paprosky and Vancouver score subgroups was compared. Clinical assessments included Harris hip scores, the fate of the grated bone and a review of complications.
Categorical variables were presented using frequency and percentages, while continuous variables were presented means and ranges. The paired t-test was used to evaluate duration of incorporation and length of strut allograft. One way ANOVA was used to compare the duration of incorporation of the cortical strut allograft by Paprosky and Vancouver subgroups. Spearman correlation coefficients were used to evaluate the length of the cortical strut allograft and assess correlation with the duration of incorporation. A P-value less than 0.05 was considered significant. Data were analyzed using SPSS Statistics software, version 19 (IBM Co., Armonk, NY, USA).
Paprosky subgroups of the 14 cases of aseptic loosening of THA or infection include type 2A (n=2), type 2B (n=5), type 3A (n=2), type 3B type (n=3), and type 4 (n=2). Of the 5 cases with periprostetic fracturs, subgroups include Vancouver type B1 (n=4) and type B3 (n=1). The average length of cortical strut allograft used in this study was 123.3 mm (35–241 mm); the length of cortical strut allograft used in patients with aseptic loosening of THA or infection was 103.9 mm (35–225 mm) and the average in those experiencing periprosthetic fractures was 177.6 mm (143–241 mm). As shown in Table 1, the cortical strut allografts used in patients with fractures was significantly longer compared with those treated for aseptic loosening or infections (P=0.013). An average of 1.4 strut bones (range, 1–3) were used for each case and applied to the fracture site in the case of periprosthetic fractures and to the bone defect site or osteotomy site in the case of loosening or infection.
The average time to incorporation, 21.2 months (range, 8–43 months) in those cases of aseptic loosening of THA or infection compared with 18.9 months (range, 13–26 months) for those experiencing periprosthetic fractures, was not significantly different (P=0.735). No positive correlation was observed between the length of cortical strut allograft and time to incorporation (P=0.494). No correlation was observed based on the time to incorporation of the cortical strut allograft and Paprosky or Vancouver subgroups, P=0.556 and 0.667, respectively (Fig. 1, 2). The average Harris hip score at final follow up was 93.3.
One revision surgery will be undertaken to treat aseptic loosening, but incorporation of the cortical strut allograft is well and there is no fracture line in the strut allograft. In another case, the hardware and cortical strut allograft were removed following a postoperative infection and the patient was discharged in resection arthroplasty state. Postoperative dislocations occurred in two cases, but there was no fracture or malunion of cortical strut allograft.
Kaplan-Meier survivorship analysis based on time of nonunion and removal of cortical strut allograft resulted in a survival rate at the final follow-up of 94.7% (Fig. 3).
Revision THAs aim to restore the stability of a THA insertion by reconstructing damaged bone, and to normalize hip joint dynamics. If there are bone defects or insufficient femoral stem support during a revision THA, a cortical strut allograft may be considered. Two types of allografts can be used during a revision THA: i) a morselized allograft for severe bone defects of the femoral bone at the graft site9), or ii) a cortical strut allograft when defects of the cortical bone prevent proper support required for artificial hip joint implants10). Cortical strut allografts can be firmly fixed to wide contact surfaces and provide sufficient bone stock by combining with the host bone; studies have shown more favorable outcomes with cortical strut allografts compared with the use of metal plates45612). In this study, revision THA plus cortical strut allografts used in patients who failed total joint replacement resulted in high bone incorporation and survival rates, similar to the results of other studies.
Lim et al.12) used an average of 147 mm of an allopathic cortical bone during revision THA in Paprosky Class 3A type patients, and Gross et al.13) used an average of 154 mm allopathic cortical bone in ORIF of patients with periprosthetic fractures. In this analysis, we note that the length of cortical strut allografts used to treat loosening THA was significantly shorter than the length of cortical strut allografts used to treat periprosthetic fractures. Subgroup analyses based on Paprosky classifications were tested, and contrary to the studies by Lim et al.12), it appears that longer cortical strut allografts are required to obtain sufficient mechanical support in cases of periprosthetic fracture vs aseptic loosening of THA or infections. Incorporation appears to depend on two factors: i) the stability of the grafted bone-host bone interface and ii) the fixation of the femoral stem. Head et al.14) reported that incorporation rates increased when the allograft bone and femoral stem were fixed with cement, and decreased when there were fractures in the allograft bone. Barden et al.15) reported that the average duration of incorporation of cortical strut allograft used in revision THA was 17 months. In our experience, cortical strut allografts were stably fixed using a control cable. In the cases of fracture and loosening, excellent incorporation rates were observed, and similar results were demonstrated by Barden et al.15) with an average of 19 months. There was no significant difference between the duration of incorporation of the cortical strut allograft used to treat loosening of THA compared with periprosthetic fractures. Similar to the results obtained here, other studies have shown that the length of the cortical strut bone and time to incorporation varied for patients. One study analyzing the impact of strut allograft length on time to incorporation has not been confirmed. Therefore, here we aimed to further analyze this relationship and showed that no positive correlation between the length of cortical strut allograft and time to incorporation was observed. Previous results suggest that when the cortical strut allograft is stably fixed, osteogenesis by osteoconduction results in similar rate in the whole bone graft. In our analysis, there was no difference in the time to incorporation of the allograft cortical bone among Vancouver and Paprosky subgroups, thus suggesting that this approach is viable regardless of fracture classification.
Cortical strut allografts can lead to problems including vascular injury of the proximal femur and infection, and are also associated with increased costs16). The rate of infections in revision THA performed with cortical strut allografts is reported to be between 0% and 2%617). Infection, a key complication associated with cortical strut allografts, was found in one patient who was discharged after removal of the THA and strut cortical bone graft and in resection arthroplasty state. In order to prevent infections, the surgical site should be thoroughly cleaned. Tomford et al.18) reported that the incidence of infection is proportional to the complexity of the operation and is not significantly affected by the presence of allograft bone. Therefore, it is mandatory to thoroughly manage the wound after surgery to prevent the spread of wound infection to deep infection.
Osteoporosis in cortical strut allografts increases over time following surgery, leading to an increased risk of fracture at the graft site. Berry et al.19) reported that the risk of graft site fracture was highest 2 to 3 years postoperatively. However, no complications (e.g., bone resorption and fracture), which commonly occur in allogeneic cortical bone grafts were reported in this study.
The failure rate of allogeneic cortical bone is 0% to 7%, showing a low morbidity rate518). Head and Malinin5) reported a 97% survival rate at a 9.5 year follow-up of patients with revision THA plus allograft bone grafts. Barden et al.3) reported a survival rate of 100% for allogeneic cortical bone at a 4.7 year follow-up. Emerson et al.10) reported a follow-up of cortical bone allografts with a survival rate of 93% after 8.4 months. Clinical results and implant device stability were all successful. In our study, the estimated Kaplan-Meier estimated survival rate (no infection, non-incorporation of cortical bone allografts, or bone removal) was 95.2% at the final follow-up.
This study has several limitations. First, the number of cases reviewed is small. There were no significant differences in fracture, loosening, and duration of each incorporation among varying classification types. This suggests that further studies with greater numbers of cases are necessary. Second, the results were interpreted by a single observer and the interpretation of time and degree of radiological bone incorporation may differ between observers. It is worth noting, however, that the individual interpreting the results has a great deal of experience in this area. The final limitation relates to image analysis. As the anteroposterior and lateral femoral x-rays were performed only at outpatient follow-ups, there may be missing radiographic transparent lines hidden by the opaque shadow between the allograft bone and the implanted devices of insertion. Overall, however, this analysis provides a thorough review of the results of medium- and long-term clinical and radiological follow-ups of cortical bone allografts during THA.
Medium- to long-term follow-up results of cortical strut allografts used in revision THA show high incorporation and survival rates. Cortical strut allograft is considered a useful approach for treating femoral periprosthetic bone defects in revision THAs and periprosthetic fractures regardless of classification.
References
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005; 87:1487–1497. PMID: 15995115.

2. Kurtz SM, Ong KL, Schmier J, et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J Bone Joint Surg Am. 2007; 89(Suppl 3):144–151. PMID: 17908880.

3. Barden B, Fitzek JG, Huttegger C, Löer F. Supportive strut grafts for diaphyseal bone defects in revision hip arthroplasty. Clin Orthop Relat Res. 2001; (387):148–155. PMID: 11400876.

4. Gross AE, Blackley H, Wong P, Saleh K, Woodgate I. The role of allografts in revision arthroplasty of the hip. Instr Course Lect. 2002; 51:103–113. PMID: 12064094.
6. Hamer AJ, Suvarna SK, Stockley I. Histologic evidence of cortical allograft bone incorporation in revision hip surgery. J Arthroplasty. 1997; 12:785–789. PMID: 9355008.

7. Paprosky WG, Lawrence J, Cameron HU. Femoral defect classification: clinical application. Orthop Rev. 1990; 19(Suppl 9):9–15.
8. Wang JW, Wang CJ. Periprosthetic fracture of the femur after hip arthroplasty: The clinical outcome using cortical strut allografts. J Orthop Surg (Hong Kong). 2000; 8:27–31.

9. Kligman M, Rotem A, Roffman M. Cancellous and cortical morselized allograft in revision total hip replacement: A biomechanical study of implant stability. J Biomech. 2003; 36:797–802. PMID: 12742447.
10. Emerson RH Jr, Malinin TI, Cuellar AD, Head WC, Peters PC. Cortical strut allografts in the reconstruction of the femur in revision total hip arthroplasty. A basic science and clinical study. Clin Orthop Relat Res. 1992; (285):35–44.
11. Head WC, Malinin TI, Mallory TH, Emerson RH Jr. Onlay cortical allografting for the femur. Orthop Clin North Am. 1998; 29:307–312. PMID: 9553575.

12. Lim CT, Amanatullah DF, Huddleston JI 3rd, Hwang KL, Maloney WJ, Goodman SB. Cortical Strut Allograft Support of Modular Femoral Junctions During Revision Total Hip Arthroplasty. J Arthroplasty. 2017; 32:1586–1592. PMID: 28130016.

13. Gross AE, Wong PK, Hutchison CR, King AE. Onlay cortical strut grafting in revision arthroplasty of the hip. J Arthroplasty. 2003; 18(3 Suppl 1):104–106. PMID: 12730942.

14. Head WC, Hillyard JM, Emerson RH Jr, Peters PC Jr. Proximal femoral allografts in revision total hip arthroplasty. Semin Arthroplasty. 1993; 4:92–98. PMID: 10148550.

15. Barden B, Ding Y, Fitzek JG, Löer F. Strut allografts for failed treatment of periprosthetic femoral fractures: good outcome in 13 patients. Acta Orthop Scand. 2003; 74:146–153. PMID: 12807320.

16. Schmidt AH, Kyle RF. Periprosthetic fractures of the femur. Orthop Clin North Am. 2002; 33:143–152. PMID: 11832318.

17. Engh CA, Massin P. Cementless total hip arthroplasty using the anatomic medullary locking stem. Results using a survivorship analysis. Clin Orthop Relat Res. 1989; (249):141–158.
18. Tomford WW, Starkweather RJ, Goldman MH. A study of the clinical incidence of infection in the use of banked allograft bone. J Bone Joint Surg Am. 1981; 63:244–248. PMID: 7007391.

19. Berrey BH Jr, Lord CF, Gebhardt MC, Mankin HJ. Fractures of allografts. Frequency, treatment, and end-results. J Bone Joint Surg Am. 1990; 72:825–833. PMID: 2365716.

Fig. 1
A 65-year-old male patient who underwent revision total hip arthroplasty (THA) with cortical strut allograft due to infection of previously existing THA. (A) Preoperative X-rays demonstrate infection near the site of a previous THA. (B) Revision THA was undertaken following measures to control infection; cortical strut allograft was performed to stabilize areas of bone defect. (C) Complete incorporation was observed after 30 months.
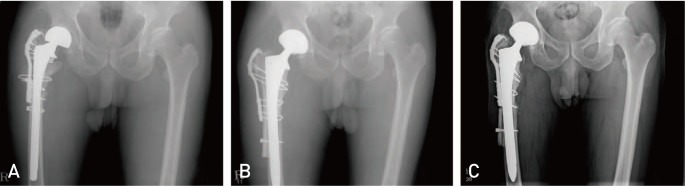
Fig. 2
A 43-year-old male patient suffering from a periprosthetic fracture resulting from a fall underwent open reduction, internal fixation and cortical strut allograft. (A) Preoperative X-ray reveals a Vancouver type B1 fracture. (B) Anterior posterior and axial views of the femur following open reduction, internal fixation and cortical strut allograft. (C) One-year postoperative X-ray showing complete incorporation.
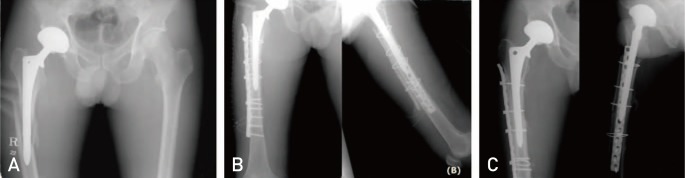
Fig. 3
Kaplan-Meyer survival curve. The survival rate at the final follow-up was 94.7% when the endpoint was set as the time of nonunion and removal of cortical strut allograft.
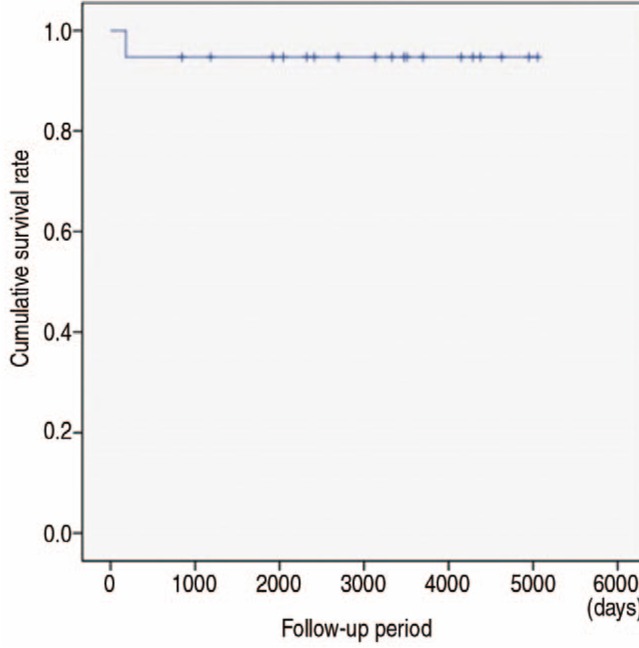
Table 1
Baseline Demographics





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download