Abstract
Purpose
The treatment of infected revision total hip arthroplasty (THA) is very challenging due to retained revision prosthesis, poor bone stock and soft tissue condition derived from previous revision surgeries, and comorbidities. The purpose of this study was to investigate the effectiveness and short-term outcomes of aggressive debridement and use of antibiotic-loaded cement beads with retention of the prosthesis for acute delayed or late infection of revision THAs.
Materials and Methods
Ten consecutive patients with symptoms or signs of less than one-week evolution and well-fixed prostheses, were treated with this procedure and a postoperative course of organism-specific antibiotics for a minimum of 6 weeks. All hips presented with acute delayed or late infection of revision THAs. Patients with a mean age of 68.1 years (range, 59-78 years) underwent an average of 1.9 previous revision THAs (1-4) before the index surgery. The minimal follow-up was 2 years with a mean of 46.2 months (range, 24-64 months).
Results
There were 8 cures (80.0%) and 2 failures with no mortality during the study period. The 2 failures involved the same and resistant bacteria implicated in the primary infection (methicillin-resistant Staphylococcus aureus and Prevotella oralis, respectively). The mean Harris hip score was 65.2 (range, 26-83) and the mean visual analogue scale was 2.6 (range, 1-4) at final follow-up.
The number of revision total hip arthroplasty (THA) is on the rise in conjunction with an extension of the average life expectancy and a corresponding increase in the frequency of primary THA1). Compared to primary THA, revision surgery is a longer and more complicated procedure involving more extensive soft tissue damage and a greater risk of periprosthetic joint infection (PJI) and dislocation, as well as other long-term complications. PJI, one of the most devastating complications of THA, requires revision surgery and poses increased risk to the patient234). PJI has been known to occur more frequently in revision THA than in primary THA, with an incidence rate of 4% to 6%, and may be more serious than in primary THA in various ways23456). Moreover, greater tissue damage and a higher risk of complications may develop as revision surgery is repeated, regardless of the causes of revision.
In general, the standard care for treatment of infected THAs is two-stage reimplantation with insertion of some type of antibiotic spacer, although various treatment options exist467). This protocol includes removal of all existing prostheses, including both the acetabular and femoral components and placement of either a static or dynamic antibiotic-loaded polymethylmethacrylate (PMMA) cement spacer. Intravenous (IV) antibiotics are administered for a minimum of 6 weeks with next delayed reimplantation. However, it is doubtful whether this method can be routinely performed in infected revision THAs considering the poor status of soft tissue and bone stock due to prior surgeries, especially in patients with comorbidities. Similarly, the treatment of infected revision THA is very challenging due to retained revision prosthesis, poor bone stock and soft tissue condition derived from previous revision surgeries.
To date, there has been little information in the literature and no consensus regarding the methods of re-revision for infected revision THAs. In this study, we present the effectiveness and short-term outcomes of aggressive debridement and use of antibiotic-loaded cement beads with retention of the prosthesis for acute delayed or late infection of revision THAs.
Between April 2008 and December 2011, we prospectively enrolled 10 consecutive patients who developed acute delayed or late PJI after at least one revision THA due to infection or other causes. Acute PJI was defined as infection presenting with symptoms or signs less than 1 week (new onset of painful hip, fever, chills, drainage or redness around the wound) in a previously well-functioning implant and developing between 3 and 24 months after surgery (delayed) or after 24 months (late) according to Zimmerli/Trampuz's classification8910).
Exclusion criteria included radiologic signs of bone infection (periosteal apposition or osteolysis around the implant) or implant loosening. The study was approved by the local ethics committee. All patients were treated with aggressive debridement and antibiotic-loaded cement beads with retention of the prosthesis and replacement of the femoral head and acetabular liner. The sample included 5 male (5 hips) and 5 female (5 hips) patients with a mean age of 68.1 years (range, 59-78 years) at time of revision surgery. The average body mass index at the time of index surgery was 27.1 kg/m2 (range, 23.2-34.2 kg/m2). The mean American Society of Anesthesiologists (ASA) score was 2.9 (range, 2-4). A summary of demographic data is provided in Table 1. These patients underwent previous revision THAs of an average of 1.9 times (range, 1-4 times). The causes of last revision surgery were infection in 5 cases, which had been treated with two-stage reimplantation, and loosening accompanied with osteolysis in 5 cases. All patients in this study had at least two medical comorbidities before the operation (Table 2).
For the femoral stems left in situ, the design was revision in 9 hips, except one hip (case 10) with primary stem, and the fixation was cementless in all hips. Meanwhile, the acetabular components were cementless metal shell in 9 hips and acetabular reinforcement ring in one hip (case 10).
The mean duration between prior revision THA and the diagnosis of PJI was 34.6 months (range, 3-87 months). All the patients were followed-up a minimum of 2 years after index surgery with a mean of 46.2 months (range, 24-64 months). No patients were lost to follow-up.
All surgeries were performed by single experienced arthroplasty surgeon. All patients in this study had removal of the acetabular liner and femoral head as well as retention of well-fixed acetabular metal shell and femoral stem. Intraoperative cultures including 5 tissue samples and one synovial fluid sample were obtained from the joint in each patient. We performed a complete sharp synovectomy, excision of all nonviable tissue (including bone and soft tissue), and mechanical cleansing with diluted povidone-iodine of exposed metal and soft tissue as part of the procedure. Antibiotic-loaded acrylic cement beads were fabricated by adding vancomycin to the pre-manufactured PMMA mixed with full-dose erythromycin/colistin (Antibiotic Simplex®P; Howmedica Osteonics Corp, Mahwah, NJ, USA) and inserted into the joint after new femoral head and acetabular liner were replaced in all patients. Generally, 4 g of vancomycin per 40 mg bag was added to the PMMA. The hip was reduced and the wound closed in a standard fashion.
All patients were evaluated and managed perioperatively by the same medical and infectious disease consultants. Microorganism-specific IV antibiotics were administered for a minimum of 6 weeks in patients with positive cultures111213). Oral antibiotics were added for resistant microorganisms as needed1213). For those who did not have positive cultures, we generally used a 6-week course of teicoplanin as directed by the infectious disease consultant. Routine weekly surveillance of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) was performed. Removal of cement beads in each patient was performed if ESR and CRP values trended downward; normal values were not required if the wound was well healed, and the minimum of 6 weeks of antibiotic therapy was completed. The entire wound was thoroughly redebrided during removal of cement beads. The infectious disease consultant remained involved following intraoperative cultures; and if cultures were positive, organism-specific antibiotics were reinitiated. After removal of cement beads, patients were seen in follow-up at 6 weeks, 3 and 6 months, one year and yearly thereafter. Clinical evaluation was performed by the treating surgeon and included the Harris hip score (HHS) and visual analog scale (VAS). At each follow-up visit, serum ESR, CRP, and radiographs were evaluated routinely. On follow-up radiographs, the presence of progressive osteolysis or loosening of prostheses including progressive radiolucent line ≥2 mm or position change of prostheses was evaluated. If there was a concern, follow-up at more appropriate frequent intervals was performed.
The criteria for the control of infection were as follows: no pain or swelling, no wound drainage, normal serology (ESR <20 mm/hr, CRP level <0.5 mg/dL, a synovial fluid white blood cell (WBC) differential of <65% neutrophils or a WBC count <1.7×103 µL), a satisfactory radiograph at least two years after the end of antibiotic therapy, and a functional THA at latest follow-up1114). Treatment failure was defined as persistence or recurrence of the symptoms or signs of PJI, the isolation of the same or different organisms from subsequent operative samples, the removal of the prosthesis while antibiotic therapy continued, or death from PJI121516).
On bacterial culture, microorganism was positively identified in 8 of 10 patients (80.0%). There was no organism identified in 2 of 10 patients (20.0%). The same organism was identified in 2 of 5 patients who underwent prior revision THA due to PJI. Gram-positive Staphylococcus species accounted for the majority of organisms cultured, present in 6 patients (60.0%), 5 of which were methicillin-resistant (50.0%) (Table 3). In the other two patients, Pseudomonas aeruginosa and Prevotella oralis (anaerobic gram-negative bacilli) were cultured in each patient, respectively. Organisms identified at the time of initial radical debridement in the 2 patients who later failed were P. oralis and methicillin-resistant Staphylococcus aureus (MRSA), respectively.
There was no clinical evidence of reinfection in 8 of 10 patients at a minimum 2-year follow-up (Fig. 1). Although CRP was eventually normalized in all eight patients in whom infection was regarded as a complete control, ESR was not completely normalized in one patient (case 2) with underlying diseases such as rheumatoid arthritis and hypothyroidism. However, infection was regarded to be completely controlled because this patient met the criteria for the control of infection except for slightly elevated ESR.
Meanwhile, there were 2 failures due to recurrence or persistence of infection implicating the primary bacterium. The first failure (case 3) concerned a 75-year-old woman with diabetes, hypertension, liver cirrhosis, ASA 4, and a history of post-stroke seizure. She underwent two-stage reimplantation THA due to MRSA infection one year after bipolar hemiarthroplasty for femoral neck fracture of the left hip. She developed recurrent PJI and intraoperative cultures positive for MRSA 20 months after reimplantation. Antibiotherapy was maintained for 8 weeks after aggressive debridement and the insertion of antibiotic-loaded cement beads. Removal of cement beads was undertaken 77 days after their insertion. However, despite two more debridements, infection was not completely controlled clinically, as seen on blood tests. The patient intermittently underwent long-term oral suppressive antibiotics considering her age and medical condition, and PJI appeared stable at 2-year follow-up. The second failure (case 10) concerned a 59-year-old woman with hypertension, peptic ulcers, depression, and ASA 2. She had already undergone three-time revision THA due to liner wear, loosening, or osteolysis after THA for secondary osteoarthritis of left hip. The patient showed abrupt hip pain and functional impairment with fever and chill after dental treatment 31 months after her last revision THA. The infection was considered probably secondary to the oral cavity associated with dental treatment in view of the type of bacterium (P. oralis) confirmed on hip aspiration. Antibiotherapy was maintained for 6 weeks after aggressive debridement and the insertion of antibiotic-loaded cement beads. Removal of cement beads was undertaken 52 days after their insertion. Relapse with sinus tract occurred one week after the end of antiobiotherapy. This patient was successfully treated with resection arthroplasty and IV antibiotics without two-stage reimplantation due to a severe acetabular bone deficiency and high risk of reinfection. At 2-year follow-up, the infection was resolved.
There was no patient death during the study period at an average of 46.2 months postoperatively. The time interval between insertion of the cement beads and removal averaged 61 days (range, 45-77 days). During the retention of cement beads in hip joints, there were no laboratory findings suggestive of nephrotoxic adverse effect of vancomycin.
The mean HHS was 65.2 (range, 26-83) at most recent follow-up and the mean VAS was 2.6 (range, 1-4) (Table 4). Nearly all of the patients ambulated either independently or with a walking aid such as a cane or crutches except one patient (case 3). On the final follow-up radiographs, all the prostheses were well-fixed without loosening in all patients except one (case 10), although bony resorption on lateral cortex of femur was partially observed in 3 patients.
Once an infection in revision THA has been diagnosed, several treatment options can be performed, similarly as in primary THA. The treatment option depends on the type of bacteria, patient sensitivity to antibiotics, duration of the infection, fixation of the hip prostheses, and the patient's overall medical condition345912151617181920212223). Which treatment method is the most effective and safest in infected revision THA remains questionable because there has been little information on the literature focused on the treatment methods of infected revision THAs. Moreover, to our knowledge, there are no reports of prospective studies of our approach of using a cohort of only infected revision THAs. In the current study, we treated acute delayed or late infection of revision THAs with aggressive debridement and antibiotic-loaded cement beads with retention of the prosthesis as an alternative, and preliminarily reported the short-term outcomes of this treatment method. We observed an 80% success rate and no deaths at a minimum 2-year follow-up, and a mean HHS of 65.2 despite the patients' comorbidities and a history of at least one revision THA.
Our study has several limitations. The first is that this study has a small number of patients preventing statistical analysis of risk factors for failure despite a cohort of only infected revision THAs, of which the treatment has been not yet addressed. This is due to a rarity of infected revision THAs, the use of strict indications to apply this technique, and difficulty in collecting a large sample size enough to have power to perform statistical analysis from one center. The second is that this study is only a prospective case series. Therefore, a randomized comparative trial from other centers with a larger number of cases is needed to confirm these results before we could recommend our procedure for wider use. The third limitation is that it is very difficult to exactly estimate the evolution of symptoms or signs as there is an occult interval between implant contamination after prior revision THA and clinical expression that may amount to several days. The fourth shortcoming of this study is that not all patients had positive cultures. Although all patients met the criteria established by the Musculoskeletal Infection Society for a PJI, an organism was not identified in two patients. However, a recent study reported that cultures often have a high false-negative rate, and culture-negative PJI is reported to occur in 7% to 9.5% of all infected arthroplasties24).
Generally, two-stage reimplantation has been used as the definite treatment for infected THAs, with a higher success rate than for other established methods. Engesaeter et al.19)reported the results of treatment of 784 infected THAs. The reported success rate was 96% for those treated with two-stage reimplantation and 92% for one-stage reimplantation. However, these extensive surgeries for treatment of infected revision THA will be difficult and risky to perform in patients with comorbidities and prior revision THAs. Furthermore, the likelihood of complications is higher in infected revision THA than in infected primary THA along with more extensive loss of muscular mass, bone, or both162325). Accordingly, the treatment of infected revision THA is challenging and the decision about which option to perform is very difficult for arthroplasty surgeons. As an alternative, less extensive surgeries such as debridement and component retention, minor partial one-stage exchange, and major partial one-stage exchange have been performed in a select group of cases, and success rates ranging from 38% to 90% have been reported to date91220212223). We performed aggressive debridement and used antibiotic-loaded cement beads with retention of the prosthesis for treating acute delayed or late infection of revision THAs, and did even in cases of reinfection after two-stage reimplantation for infected primary or revision THAs because a low success rate of 36.4% has been reported after another reimplantation, to date25). Our preliminary study shows a favorable result compared with other less extensive surgeries despite a lower success rate than with one- or two-stage reimplantation1920212223). We believe that our modified procedure is a less extensive but more effective method in terms of local delivery of antibiotics and maintenance of high concentration using antibiotic-loaded cement beads in relatively poor soft tissues and bone stock due to at least two hip arthroplasties. However, preoperatively, we should mention to the patients and their families about the possibility of the failure of this procedure and the extra cost and morbidity of subsequent operations.
We put forward the following hypotheses about the causes of the two failures in our study. In the first case of failure, the causes may have been advanced age in an ASA 4 patient, an acute recurrent episode in a chronic PJI in immunocompromised patient due to comorbidities, and resistant microorganism2223). In the second case, the patient had previously undergone three-time revision THAs, and had poor soft tissue condition and bone stock. The other causes may have been heavy metal materials positioned on the acetabular side and insufficient debridement due to those, a rare and resistant anaerobic microorganism due to acute hematogenous dissemination secondary to oral contamination, and comorbidities.
Mortality associated with two-stage reimplantation of hip PJI appears to be high both in the perioperative period and within the follow-up interval15). Toulson et al.18) reported a 26% rate of death before 2-year follow-up in their series and Ekpo et al.16) reported an overall mortality rate of 45% during the study period. In the current study, there was no death at an average of 46.2 months after surgery as well as perioperatively or within 90 days. This suggests that this procedure is relatively safe even in infected revision THAs in patients with comorbidities. However, the current study has too small a sample size to generalize this mortality rate and directly compare it with the results from previously published studies, despite the prospective data.
Radical debridement and usage of antibiotic-loaded cement beads with retention of well-fixed prostheses for treating acute delayed or late infection of revision THAs shows a favorable success rate and no mortality in patients with comorbidities. Accordingly, this treatment method may be a safe and effective alternative for acute delayed or late infection of revision THAs, especially when more extensive surgery is difficult to perform due to several causes such as advanced age and comorbidities.
References
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005; 87:1487–1497. PMID: 15995115.

2. Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005; 87:1746–1751. PMID: 16085614.

3. Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999; 48:111–122. PMID: 10098033.
4. Salvati EA, González Della, Masri BA, Duncan CP. The infected total hip arthroplasty. Instr Course Lect. 2003; 52:223–245. PMID: 12690851.
5. Sanchez-Sotelo J, Berry DJ, Hanssen AD, Cabanela ME. Midterm to long-term followup of staged reimplantation for infected hip arthroplasty. Clin Orthop Relat Res. 2009; 467:219–224. PMID: 18813895.

6. Spangehl MJ, Younger AS, Masri BA, Duncan CP. Diagnosis of infection following total hip arthroplasty. Instr Course Lect. 1998; 47:285–295. PMID: 9571430.
7. Hanssen AD, Spangehl MJ. Treatment of the infected hip replacement. Clin Orthop Relat Res. 2004; (420):63–71.

8. Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection. Bone Joint J. 2013; 95-B:1450–1452. PMID: 24151261.

9. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996; 78:512–523. PMID: 8609130.

10. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004; 351:1645–1654. PMID: 15483283.

11. Kim YH, Kim JS, Park JW, Joo JH. Cementless revision for infected total hip replacements. J Bone Joint Surg Br. 2011; 93:19–26. PMID: 21196538.

12. Aboltins CA, Page MA, Buising KL, et al. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007; 13:586–591. PMID: 17331125.

13. Cataldo MA, Petrosillo N, Cipriani M, Cauda R, Tacconelli E. Prosthetic joint infection: recent developments in diagnosis and management. J Infect. 2010; 61:443–448. PMID: 20932998.

14. Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976; (117):221–240. PMID: 776484.

15. Berend KR, Lombardi AV Jr, Morris MJ, Bergeson AG, Adams JB, Sneller MA. Two-stage treatment of hip periprosthetic joint infection is associated with a high rate of infection control but high mortality. Clin Orthop Relat Res. 2013; 471:510–518. PMID: 22983683.

16. Ekpo TE, Berend KR, Morris MJ, Adams JB, Lombardi AV Jr. Partial two-stage exchange for infected total hip arthroplasty: a preliminary report. Clin Orthop Relat Res. 2014; 472:437–448. PMID: 23852737.

17. Oussedik SI, Dodd MB, Haddad FS. Outcomes of revision total hip replacement for infection after grading according to a standard protocol. J Bone Joint Surg Br. 2010; 92:1222–1226. PMID: 20798438.

18. Toulson C, Walcott-Sapp S, Hur J, et al. Treatment of infected total hip arthroplasty with a 2-stage reimplantation protocol: update on “our institution's” experience from 1989 to 2003. J Arthroplasty. 2009; 24:1051–1060. PMID: 18848425.
19. Engesaeter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register. Acta Orthop. 2011; 82:530–537. PMID: 21992085.
20. Estes CS, Beauchamp CP, Clarke HD, Spangehl MJ. A twostage retention débridement protocol for acute periprosthetic joint infections. Clin Orthop Relat Res. 2010; 468:2029–2038. PMID: 20224958.

21. Tattevin P, Crémieux AC, Pottier P, Huten D, Carbon C. Prosthetic joint infection: when can prosthesis salvage be considered? Clin Infect Dis. 1999; 29:292–295. PMID: 10476729.

22. Meehan AM, Osmon DR, Duffy MC, Hanssen AD, Keating MR. Outcome of penicillin-susceptible streptococcal prosthetic joint infection treated with debridement and retention of the prosthesis. Clin Infect Dis. 2003; 36:845–849. PMID: 12652384.

23. Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006; 42:471–478. PMID: 16421790.

24. Huang R, Hu CC, Adeli B, Mortazavi J, Parvizi J. Culturenegative periprosthetic joint infection does not preclude infection control. Clin Orthop Relat Res. 2012; 470:2717–2723. PMID: 22733184.

25. Kalra KP, Lin KK, Bozic KJ, Ries MD. Repeat 2-stage revision for recurrent infection of total hip arthroplasty. J Arthroplasty. 2010; 25:880–884. PMID: 20206469.

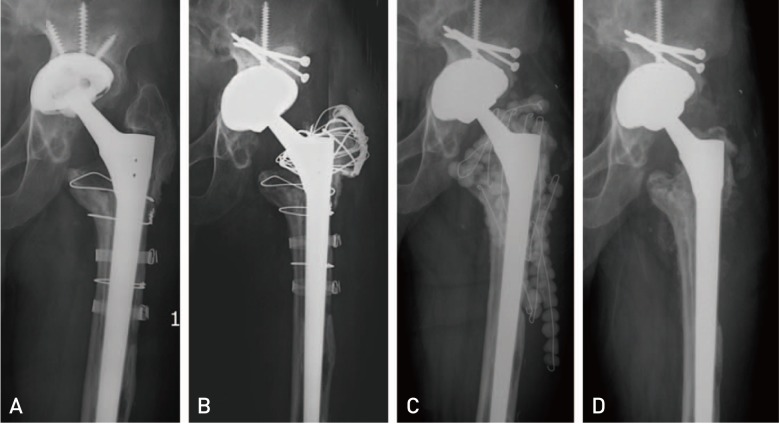
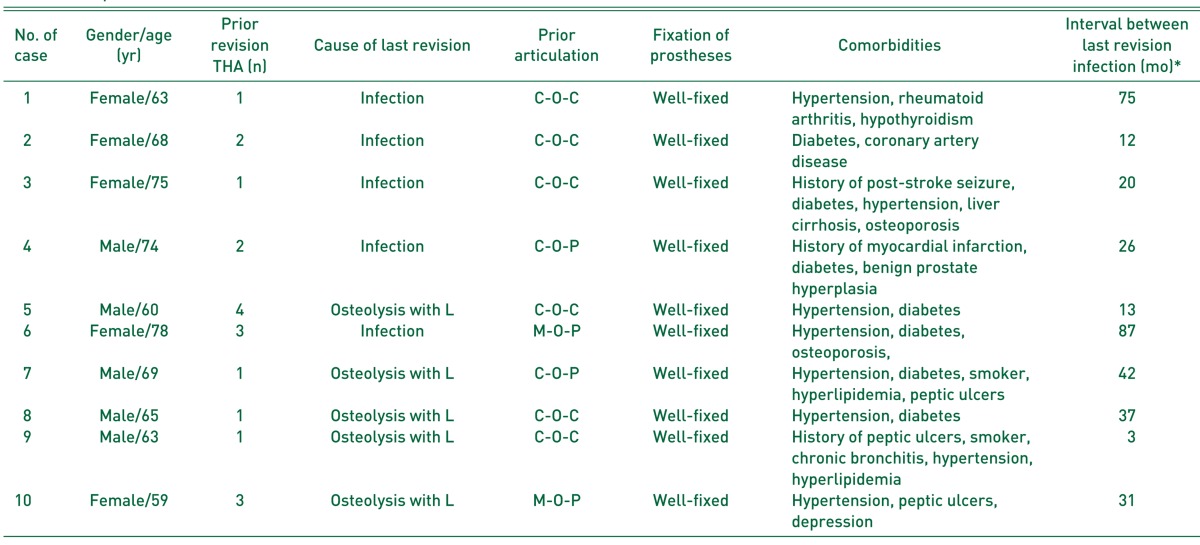
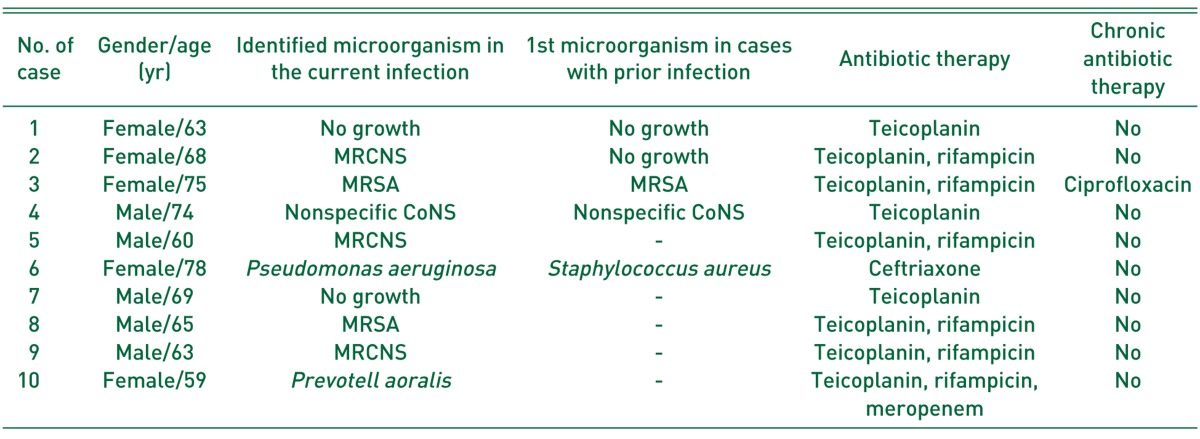
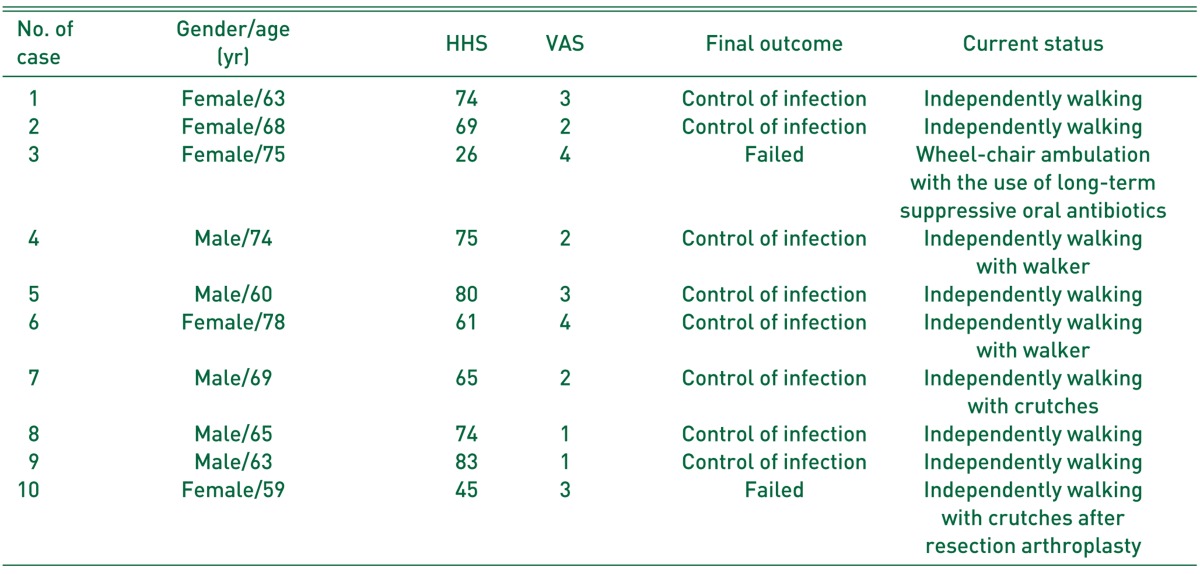
Fig. 1
Anteroposterior radiograph of a 60-year-old man (case 5), showing acetabular cup loosening accompanied with osteolysis after three-time revision total hip arthroplasties (THAs) due to liner wear, osteolysis, or loosening for 20 years (A). (B) Periprosthetic joint infection developed 13 months after fourth revision THA, which was confirmed on the culture of hip aspirate. (C) Aggressive debridement and insertion of antibiotic-loaded cement beads with retention of prostheses, removal of some metal materials, and replacement of femoral head and acetabular liner, were performed. (D) Infection was controlled and left hip was well-functioned with Harris hip score of 80 points 26 months after 6-week antibiotics treatment and removal of cement beads with repetitive debridement, although there was some bony resorption on the lateral cortex of proximal femur.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download