Abstract
Rhabdomyolysis is most frequently caused by soft tissue injury with trauma to the extremities. Non-traumatic rhabdomyolysis may be caused by alcohol or drug abuse, infection, collagen disease, or intensive exercise, but incidence is low. In particular, rhabdomyolysis resulting from carbon monoxide poisoning is especially rare. If caught before death, carbon monoxide poisoning has been shown to cause severe muscle necrosis and severe muscle damage leading to acute renal failure. In cases of carbon-monoxide-induced rhabdomyolsis leading to acute compartment syndrome in the buttocks and sciatic nerve injury are rare. We have experience treating patients with acute compartment syndrome due to rhabdomyolysis following carbon monoxide poisoning. We report the characteristic features of muscle necrosis observed during a decompression operation and magnetic resonance imaging findings with a one-year follow-up in addition to a review of the literature.
Until the 1980s, carbon monoxide poisoning was very common in Korea due to the household use of charcoal briquettes for heating and cooking. The number of cases of carbon monoxide poisoning has dramatically decreased with the distribution of alternative fuels. However, suicides by carbon monoxide poisoning are occasionally encountered in the emergency room. One of the complications associated with carbon monoxide is carbon monoxide toxicity on skeletal muscle. This may cause skeletal muscle necrosis and necrotized muscles could lead to acute renal failure due to rhabdomyolysis1). Rhabdomyolysis caused by carbon monoxide poisoning and acute compartment syndrome inducing sciatic nerve injury are very rare in patients without trauma to the buttocks. We report the clinical manifestations and magnetic resonance imaging (MRI) findings of rhabdomyolysis, operative findings, and treatment of muscle necrosis observed during decompression.
A 32-year old male patient in a semiconscious state following an attempted suicide by carbon monoxide exposure was admitted to our emergency room by 119 rescue service. At the time of emergency department admission, laboratory test results revealed elevated levels of myoglobin (>1,000.00 ng/mL; normal, 0-110 ng/mL), creatine kinase (CK)-MB (264 ng/mL; normal, 0.0-5.0 ng/mL), CK (99,053 IU/L; normal, 56-244 IU/L), blood urea nitrogen (BUN; 57.5 mg/dL; normal, 8.0-20.0 mg/dL), creatinine (4.8 mg/dL; normal, 0.6-1.2 mg/dL), lactate dehydrogenase (11,200 IU/L; normal, 200-400 IU/L), C-reactive protein (CRP; 44.47 mg/dL; normal, 0-0.5 mg/dL), white blood cells (14.54×103µL; normal 4.0×103-11×103/µL) and erythrocyte sedimentation rate (43 mm/hour; normal 0-10 mm/hr). On urinalysis, urine appeared dark brown and showed erthrocyte 4+ and myoglobinuria. Urine output was less than 50 mL/hour. As the patient was unable to urinate, an increased dose of furosemide (80 mg) was administered; however, urine output remained less than 50 mL/hour. On the second day after medication, BUN and creatinine increased to 91.1 mg/dL and 8.3 mg/dL, respectively. Physical examination revealed muscle strength of Gr 1 in the right extensor hallucis longus and extensor digitorum muscles and plantar flexor and extensor muscles, loss of plantar reflex, and weakened sensation of the tibial and peroneal nerve innervation region. Severe swelling, tenderness and intense pain occurred upon passive flexion of the right buttock. The patient was diagnosed with sciatic nerve palsy associated with rhabdomyolysis, acute renal failure and compartment syndrome due to carbon monoxide poisoning.
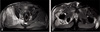
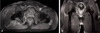
To identify the distribution and extent of muscle damage caused by rhabdomyolysis, gluteal and femoral MRI were performed. The results of MRI were as follows: i) no enhancement in the gluteal and femoral regions (T1 scan), and ii) heterogenous high signal intensity in the right gluteus maximus, medius and minimus (T2 scan). In particular, severe swelling was confirmed in the right gluteus medius and minimus, and the likelihood of compartment syndrome was considered high due to increased tissue pressure. Rhabdomyolysis was suspected as gadolinium-enhanced T1 weighted images showed peripheral enhancement of muscle and subcutaneous fat (Fig. 1, 2, 3).
Although conservative treatment was an option, surgical decompression was performed immediately, as the benefits of this approach appeared to outweigh the risk of infection and other potential complications, in light of the patient's condition (e.g., severe pain, increasing swelling in the buttock, sciatic nerve palsy and hemodialysis due to renal failure). An 18-cm incision was made in the gluteus using a posterior approach, and the tensor fascia lata around necrotic muscles was incised with Metzembaum scissors. Fasciotomy was performed to decompress the gluteal maximus compartment by spreading apart with fingers the border between the gluteus maximus and medius apart along the direction of muscle fibers.
Surgical outcomes confirmed the presence of necrotic muscle tissue. Bleeding was observed in the dermis and subcutaneous fat, but the muscle was pale and had no bleeding. No contractility was present as a response to electrical stimulation and there was no muscle elasticity. Muscle tended to be easily scraped off using the curette (Fig. 4).
Debridement and lavage were performed at the site of necrosis. Because of the extensiveness of necrosis, complete excision of necrotic muscles was not possible. Necrotic muscles that were easily removed were partially excised. Hemovac (H/V) was inserted and then the wound was closed.
Following decompression, pain was alleviated and acute renal failure improved after 7 days of daily hemodialysis. On the 21st day after admission, BUN/creatinine ratio was normalized to 10.8/1.2 mg/dL and myoglobin level was restored to 81.38 ng/mL. Necrotic tissues were consistently drained via H/V.
CRP returned to normal levels on the 7th week following admission and the wound was recovered 3 weeks after surgery. A walking aid was used starting from 4 weeks after surgery and wound healing and rehabilitation was consistently performed. The patient was discharged at 12 weeks after admission. Progressive rehabilitation was continued after hospital discharge. On physical examination at 1 year after admission, muscle strength was improved to Gr IV in the extensor hallucis longus and extensor digitorum muscles and plantar flexor and extensor muscles. Although motor ability was not fully recovered to normal level and claudication slightly remained, pain disappeared. On one-year MRI follow-up, muscle necrotic areas still remained on gadolinium-enhanced T1-weighted images.
Larrey et al. reported the first case of muscle necrosis associated with carbon monoxide poisoning in 1806, and suggested that severe muscle damage could lead to acute renal failure. Bywaters and Beall demonstrated that myoglobin released from damaged muscle cells played an important role in acute renal failure. Mautner was the first to show that rhabdomyolysis causes acute renal failure following carbon monoxide poisoning. One complication associated with carbon monoxide is its direct toxicity effect on skeletal muscle. Though myoglobin's affinity for carbon monoxide is lower than hemoglobin's for oxygen, myoglobin-bound carbon monoxide tends to accumulate in skeletal muscle tissue because of a weak disassociation property.
In carbon monoxide poisoning, local muscle compression caused by the patient's own weight increases pressure within a muscle compartment leading to swelling and muscle ischemia. As this pressure continues to rise, carboxyhemoglobin production increases and the supply of oxygen decreases leading to skeletal muscle necrosis and eventually rhabdomyolysis2). Rhabdomyolysis is the release of intracellular components into the extracellular environment and plasma following skeletal muscle damage. The majority of patients with rhabdomyolysis were associated with acute renal failure. Acute renal failure may be caused by the release of myoglobin, hemoglobin and other substances from muscle and red blood cells and likely the result of their direct toxicity on tubular epithelial cells or the formation of renal tubular precipitates; occurring at a frequency of between 5% and 8.6%3).
In a review of 250 cases, Kim et al.4) found that the leading cause of rhabdomyolysis was traumatic liver injury including traffic accident (accounting for 61.6% of all causes). Non-traumatic causes were alcohol overdose (6%), systemic convulsion (5.6%), shock, metabolic dysfunction, infection, carbon monoxide poisoning (3.2%), and snake bites (2%). Of the 68 cases of non-traumatic rhabdomyolysis reported by Kang et al.5), carbon monoxide poisoning accounted for only 1.5%. Of the 98 cases of carbon monoxide poisoning reported by Jang et al.6), the most common complication was rhabdomyolysis (in 31.6% of the cases). Most patients with acute renal failure resulting from carbon monoxide poisoning also experienced.
Thus, carbon monoxide-induced muscle necrosis may lead to rhabdomyolysis-one of the most common causes of acute renal failure. Compartment syndrome resulting from rhabdomyolysis associated with non-traumatic causes is a very rare condition. A single case of sciatic nerve palsy complicated with compartment syndrome caused by alcohol and antidepressant-induced rhabdomyolysis has been reported in Korean literature. However, rhabdomyolysis-associated compartment syndrome resulting from carbon monoxide poisoning has not yet been reported. Local body weight-induced muscle compression in a patient with carbon monoxide poisoning elevates pressure within a muscle compartment as capillary permeability and tissue pressure (resulting from the extravascular accumulation of tissue fluid) increase. Impaired oxygen supply to tissues following the formation of carboxyhemoglobin induced by muscle ischemia causes skeletal muscle necrosis; gluteal compartment syndrome occurs when excessive pressure builds up inside a closed muscle compartment. In this case report, muscle necrosis resulting from carbon monoxide poisoning appeared to be accelerated because severe local compression in the right buttock extensively broadened muscle necrosis in the right gluteal region and tissue pressure was elevated to a greater extent. Surgical findings revealed that necrosis progressed more severely in the muscle compared to the dermis and subcutaneous fat due to local compression (Fig. 4).
Owen et al.7) divide the gluteal compartments into the gluteus maximus, medius/minimus and tensor fascia lata. Gluteal compartment syndrome may occur in one or more compartments. They report that 3 patients recovered from compartment syndrome with conservative treatment (1 case) and immediate decompression (2 cases with an intracompartmental pressure exceeding 30 mmHg). In this case report, the author regrets neglecting to measure intracompartmental pressure because urgent surgical decompression was performed.
The wick catheter and slit catheter techniques have been introduced to measure intracompartmental pressure and recent intracompartmental pressure monitoring systems include the Synthes compartment pressure monitor (Synthes, West Chester, PA, USA) and the Stryker compartment pressure monitor (Stryker Orthopaedics, Kalamazoo, MI, USA).
It still remains unclear whether carbon monoxide-associated sciatic nerve palsy is caused by the toxicity of necrotic tissue or compartment syndrome. However, extensive high signal intensity was visualized on T2-weighted images of the gluteus maximus, medius and minimus muscles. In particular, severe swelling was detected in the right gluteus medius and minimus, but MRI also demonstrated severe edema in the perineural area but not high signal intensity of the sciatic nerve. On follow-up examination, muscle strength was improved to Gr IV in the extensor hallucis longus and extensor digitorum muscles and plantar flexor and extensor muscles. There is a higher likelihood of sciatic nerve injury as a result of compartment syndrome.
MRI is useful in diagnosing rhabdomyolysis and identifying the distribution and extent of muscle damage8). MRI findings are typically seen as high signal intensity on T2 weighted images and no change in signal intensity on T1 weighted images. Even though the mechanism of signal change on T2 weighted images has not yet been clarified, signal intensity changes are suggested to be caused by edema, swelling of muscle cells and interstitial inflammation occurring in the acute phase of rhabdomyolysis9). When using MRI, changes in signal intensity correlate with the degree of muscle injury. Changes in signal intensity then disappear in proportion to clinical recovery after minor injury.
In many cases, non-enhanced T1 and T2-weighted image findings are normal in subacute to chronic stages, and lesions are better seen on gadolinium-enhanced T1-weighted images. First, if the primary mechanism of gadolinium enhancement is leakage of gadolinium-enhanced fluid through inflamed blood vessels, lesions will thus be enhanced in the acute stage. Second, if the primary mechanism of gadolinium enhancement is gadolinium leakage through immature blood vessels in the angiogenic-mediated healing process, lesions will be enhanced either in subacute or chronic stage. When lesions are clearly seen on T2-weighted scans, gadolinium-enhanced MRI may be unnecessary. Gadolinium-enhanced T1 weighted imaging is a useful diagnostic tool when lesions are undetected on T2-weighted scans in cases of minor changes to inflammation in chronic stage10). Furthermore, Gadolinium-enhanced MRI is projected to be helpful in differential diagnosis of irreversible muscle necrosis and when deciding between treatment options, since complete muscle necrosis due to vascular occlusion is not enhanced while the marginal area with blood supply is only enhanced. In this case report, MRI follow-up findings revealed muscle necrotic areas still remaining on gadolinium-enhanced T1 weighted images.
Figures and Tables
Fig. 1
(A) Axial T1-weighted image shows no enhancement. (B) Coronal T2 weighted image shows heterogenous high signal intensity in the right gluteus maximus, medius, minimus.

Fig. 2
Axial T2-weighted images show heterogenous high signal intensity in the right gluteus maximus, medius, and minimus. Swelling is especially apparent in the right gluteus medius, and minimus muscles.
References
1. Lee HY, Lee KH, Han JS, Ahn CR, Kim SG, Lee JS. The Tc-MDP bone scan in the cases of acute renal failure due to carbon monoxide poisoning. Korean J Nephrol. 1985; 4:56–67.
2. Chung KJ, Chung YK, Yoo JH, Wang JS. Sciatic nerve palsy complicating gluteal compartment syndrome due to rhabdomyolysis: a case report. J Korean Orthop Assoc. 2005; 40:103–106.

3. Thomas MA, Ibels LS. Rhabdomyolysis and acute renal failure. Aust N Z J Med. 1985; 15:623–628.
4. Kim HY, Choi SO, Shin SJ, et al. Analysis of 250 cases of rhabdomyolysis. Korean J Nephrol. 1994; 13:810–817.
5. Kang SW, Kim YW, Kim YH. Analysis of nontraumatic rhabdo myolysis during recent 2 years. Korean J Med. 2004; 67:467–474.
6. Jang SW, Jeon JC, Choi WI. Risk factors associated with complications of carbon monoxide poisoning. J Korean Soc Clin Toxicol. 2009; 7:10–18.
7. Owen CA, Woody PR, Mubarak SJ, Hargens AR. Gluteal compartment syndromes: a report of three cases and management utilizing the Wick catheter. Clin Orthop Relat Res. 1978; (132):57–60.
8. Jung KC, Kwon ST, Cho KH, Kang SK, Kim JM. MR findings of acute rhabdomyolysis: case report. J Korean Radiol Soc. 2003; 49:119–123.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download