Abstract
Purpose
The results of ceramic-on-ceramic (CoC) bearing surfaces in primary total hip arthroplasty (THA) were well known. However, it was not known in revision THA. The purpose of this study is to report the results of revision THA with ceramic articulation.
Materials and Methods
A total of 112 revision THAs were evaluated. The mean age at the time of surgery was 51.6 years (27.7 to 84.2 years). The mean duration of the follow-up periods was 6.3 years (2.3 to 11.4 years).
Results
The Harris hip scores improved from an average of 56.2 at the index surgery to an average of 93.3 at the last follow-up (P<0.001). None of hips showed osteolysis or ceramic head fracture. One hip showed aseptic loosening in the acetabular component with squeaking that caused a re-revision. There were nine cases of dislocation. The survivorship at 5 years was 94.5% (95% confidence interval, 87.9% to 97.6%) with revision for any reason as the endpoint and 100% with femoral revision.
Revision total hip arthroplasty (THA) has been increasingly performed because of an overall increase of primary THA in past three decades and their limited long-term survival12). Polyethylene wear-induced osteolysis is the most significant primary factor limiting the life span of total joint arthroplasty. To reduce ultra-high-molecular-weight polyethylene particulate wear debris, highly cross-linked polyethylene (HXLPE) bearings have been introduced in THA3). The revision technique and materials continue to develop with the increase of revision THAs. Metal/ceramic-on-HXLPE, metal-on-metal, or ceramic-on-ceramic (CoC) articulations are the current bearing materials for revision THA. However, HXLPE bearing still has a polyethylene wear problem, which leads to subsequent osteolysis45). Metal ions from metal-on-metal bearing may influence kidney function and metallosis from bearing surface may induce hypersensitivity67). Ceramics as bearing surface material are very hard and smooth. These characteristics could help to decrease the amount of wear within the implants. Furthermore, CoC articulations have good mechanical and biological properties such as low wear rates and bioinertness, respectively, thereby resulting in low incidence of osteolysis. In addition, the implant-carrying periods are being prolonged, since revision THAs are being performed in younger patients than before and life-spans of patients have been prolonged. Therefore, CoC articulation could be an attractive option in revision THAs, although it has some limitations such as a short neck length and lack of diversity in the models of acetabular cup, and fracture of the latest generation ceramic heads may cause taper damage that necessitates stem removal8). However, few studies have been performed on younger patients who had undergone revision THAs using ceramic bearings, and only a small number of younger patients were enrolled in those studies. Therefore, we evaluated the clinical and radiological outcomes of revision THA using the third- and fourth-generation CoC articulations in younger patients for intermediate-term durations.
One-hundred twelve revision THAs in 103 patients were performed by single surgeon using CoC articulation from January 2000 to April 2012. All patients were available for follow-up for more than two years. The average follow-up period was 6.3 years (range, 2.3 to 11.4 years). There were 69 men and 34 women. The mean age at the time of surgery was 51.6 years (range, 27.7 to 84.2 years). The average body mass index (BMI) of the patients were 23.6 kg/m2 (range, 21.3 to 25.5 kg/m2). The reason for the primary THAs were osteonecrosis of femoral head in 57, a femur fracture in 31, coxarthrosis due to sequelae of developmental dysplasia of the hip in eight, ankylosis in four, septic arthritis sequelae in six, coxarthrosis due to sequelae of Legg-Calvé-Perthes disease in four and primary osteoarthritis in two hips. The reasons for revision surgery were osteolysis with loosening of the cup in 46, osteolysis with loosening of the stem in 11, osteolysis with loosening of both cup and stem in 13, osteolysis without loosening of the prosthesis in 30, infection in eight, and recurrent dislocation in four hips, respectively (Table 1). In this study re-revision cases were included in the revision THAs. Acetabular bone defect was graded in type I in 7, type IIA in 42, type IIB in 36, type IIC in 18, type IIIA in two and type IIIB in seven according to Paprosky's classification preoperatively. There were 68 of type I, 38 of type IIA, and six of type IIIA femoral bone defects, preoperatively9) (Table 2).
Third generation ceramic bearings, Biolox® Forte (CeramTec, Plochingen, Germany) with titanium sleeve and Duraloc option cups (DePuy, Warsaw, IN, USA) were implanted in 49 hips until June of 2007. After then, the fourth generation ceramic, Biolox® Delta (CeramTec) was used with Pinnacle cups (DePuy) in 63 hips. The cups were titanium alloy and were assembled with a ceramic liner like secure-fit10). A 36 mm head and a medium-length neck were used most frequently. All the size of head, neck and the generation of ceramic are shown in Table 3. The average of cup size was 55.8 mm (range, 48 to 58 mm). The acetabular component and head were revised with the femoral stem left in situ in 20 hips, most of which showed intact taper without corrosion. The other 92 hips were revised to CoC bearings with an exchange of all components (46 hips with AML® stems [DePuy] and 46 hips with Solution™ stems [DePuy]).
In all patients, the senior surgeon (K.H.M.) performed surgery through a posterolateral approach. Allogenous bone was grafted in 96 hips: morselized graft in 87 hips (type IIA in 38, type IIB in 32, and type IIC in 17 hips) and structural graft in nine hips (type III in 9 hips). When we tried to remove femoral component, stems were easily extracted because of loosening. Except those above, removal of well fixed stem can be achieved by extended femoral trochanteric osteotomy procedure. Allogenous femoral cortical strut bone was grafted in 73 hips (type I in 34, type II in 33, and type III in 6 hips).
For six weeks after the revision surgery, abduction of the hip joint was allowed, however, adduction, internal rotation, and more than 90° of flexion were avoided. The patients were not allowed to weight bearing for first six weeks. Partial weight bearing with crutches was allowed at six weeks after the surgery. Weight bearing was gradually increased for up to three months using crutches and walking aids as a routine protocol. Unless a complication had developed, full weight bearing was allowed after three months. Patients were recommended to be followed up at three months, six months, and one year after surgery, and every year thereafter.
Clinical evaluation included the Harris hip scores (HHS)11), questionaires regarding squeaking, limping, thigh pain, and the need for walking aids, and the measurement of clinical leg length. HHS were evaluated before revision surgery and at the last follow-up of each patient (2.3 to 11.4 years). The cases with more than 90 points were classified as excellent, between 80 points and 90 points as good, between 70 points and 80 points as fair and lower than 70 points as poor12). A questionnaire regarding squeaking or hip noise was obtained at outpatient clinic by asking patients if funny sounds had developed from the side of the operated hip when walking, ascending stairs, walking down the stairs, sitting down and bending over to pick something up. Clinical leg length was measured directly by using tapes from the anterior superior iliac spine to the medial malleolus.
Radiographic evaluations included osteolysis131415), radiolucency16), stability of component1718), graft incorporation, anteversion and inclination angles of acetabular component, and femoral offset19202122), and leg length discrepancy (LLD)23).
The evidence of osteolysis or loosening was evaluated at the last follow-up. Radiolucencies at the bone-socket interface were classified using three zones as described by DeLee and Charnley17). Regarding to stability of component, the cup was defined a loosened cup if we noted ≥2 mm migration, ≥5° changes in tilting, shedding of metal particles, and a continuous periacetabular radiolucent line. A stem was defined as loosened stem if we noted: more than 5 mm progressive subsidence, pedestal formation, a continuous radiolucent line around the stem, or the shedding of metal particles. Graft incorporation was assessed by the disappearance of the radiolucent line between graft and host bone and the remodelling of the inner structure of the bone graft. Inclination and anteversion of the acetabular component, and femoral offset were measured by two physicians.
A 'radiographic' LLD was measured by calculating the difference in vertical distance between a horizontal line drawn along the bottom of the ischial tuberosities and the most inferior points of the lesser trochanter in anteroposterior view of pelvis X-ray. All measurements were done using measurement tools in a Picture Archiving and Communication System (PACS) console (Maroview; Marotech, Seoul, Korea).
With regard to the complications, dislocation was diagnosed according to the clinical symptoms, physical examinations and radiologic findings such as X-rays and computed tomography scans. Periprosthetic joint infection was found based on the findings of synovial fluid white blood cell counts, erythrocyte sedimentation rates, and C-reactive protein. Incidences of hip dislocation and head fracture after revision were also evaluated.
A paired t-test was used to compare the pre-operative and post-operative HHSs. A Wilcoxon rank sum test was used to compare variables (age, BMI, height, inclination and anteversion) between patients with and without dislocation. A Fisher's exact test was used to compare variables (head size, generation of ceramic and gender of patients) between patients with and without dislocation. Survival analysis was performed with the use of the Kaplan-Meier method24). A P-value of <0.05 was considered significant. Statistical analysis was performed with IBM SPSS Statistics version 19.0 (IBM Co., Armonk, NY, USA).
The average HHS improved from 56.2 points (range, 42-66.2 points) preoperatively to 93.3 points (range, 82-100 points) at the last follow-up (P<0.001). As regards the grading according to the HHS, 66 (58.9%) hips belonged to 'excellent', 24 (21.4%) to 'good', 16 (14.3%) to 'fair' and 6 (5.4%) to 'poor'. The third-generation ceramic had an average improvement of 12.3 (from 4 to 35), and the fourth-generation ceramic had an average improvement of 12.0 (from 2 to 29). There was no significant difference regarding improvement of HHS between the third- and the fourth-generation ceramics (P=0.561).
Squeaking was reported in one hip (0.9%). Fifty-one (45.5%) patients walked with a limp postoperatively. Three (2.7%) patients required walking aids postoperatively. None of the patients experienced thigh pain postoperatively. The average of clinical LLD was 0.21 cm (range, 0 to 2 cm).
At the last follow up, there was no radiological sign of osteolysis around the revised prosthesis. The mean angle of inclination of the new acetabular component was 33.8° (range, 28.8° to 63.1° ) and the mean angle of anteversion of cup 21.8° (range, 17.5° to 26.0°).
There was one loosening of cup (Fig. 1). There was no loosening of femoral stem. Graft was incorporated in all the patients within twelve months after operation, according to serial radiographs, except the collapsed structural bone in the case of loosening.
The average of the femoral offset of dislocation group was 48.7 mm (range, 46.4 to 52.1 mm); whereas, non-dislocation group was 46.4 mm (range, 34.5 to 53.4 mm). There was no significant difference regarding femoral offset, head size, generation of ceramic, gender, age, or BMI between patients with and without dislocation (Table 4). The average of radiologic LLD was 0.2 cm (range, 0 to 2 cm).
The rate of dislocation was nine of 112 (8.0%). They were treated satisfactorily by a closed reduction under general anesthesia. A case of dislocation which had developed following the revision THA with the use of a constrained liner was successfully treated by changing stem/cup and using a larger head (Fig. 2). In that case we had at first employed a constrained liner since the hip had shown recurrent dislocation.
There were two prosthetic joint infections. One hip underwent cup re-revision due to loosening and two cups were re-revised with retaining femoral component due to periprosthetic joint infection at 11 months and 66 months postoperatively. Both hips had a 36 mm Biolox® Delta ceramic head placed with a 52 mm and 60 mm Pinnacle cup, respectively.
The survivorship at 5 years was 94.5% (95% confidence interval, 87.9 to 97.6) with revision for any reason as the endpoint, and 100% with femoral revision (Fig. 3).
In Asians, high incidence of osteonecrosis of the femoral head (ONFH) in a young age group correlates with a high proportion of young and physically active primary THA patients25). The age of patients who had undergone revision in this study averaged 51.6 years, 82 of 112 hips (73.2%) being revised when the patient's age was under 60 years. This group of relatively young and active patients carries a risk for subsequent revision due to their long life expectancy.
In spite of advantages of ceramics such as low wear and biologic response, there are concerns such as the third body wear resistance, larger head compared to PE, difficulty in the use of elevated liner, limitations in the use of cups and so on. We used third- and fourth-generation CoC articulations to achieve the advantages of CoC itself.
Squeaking was found in a patient who underwent re-revision due to cup loosening. The patient had cup malposition resulting from instability. We believe that the rarity of squeaking in our study was owing to our having done best to obtain proper inclination and anteversion angle and to confirm whether squeaking occurred by moving the hip joint intraoperatively.
There was one case (0.9%) of aseptic loosening of a cup in this study. Only the acetabular component showed loosening, and no sign of loosening was observed around the femoral component. Hooper et al.26) reported at 10 years of follow up, 0.4% (femur) and 0.4% (acetabulum) of component loosening was observed. Higher mechanical stress on acetabulum than on femur has been regarded as a higher rate of acetabular component failure in hip replacement27). Jack et al.8) reported two cases (1.59%) of cup loosening, the cause of which was the collapse of the structural bone graft, which replaced the extensive acetabular bone loss. Performing bone grafting only could not provide enough structural support in Paproski type III defect. In our study, CoC articulation had the disadvantage of not being able to be used with anti-protrusio cage. In addition, we could not use tantalum augmentation, since it was not available in Korea during the study period when revision was performed. As a result, enough mechanical stability could not be attained in our bone grafting.
Dislocation is the most common complication of revision THA. Chang et al.28) reported higher dislocation rate (22%) after revision THA. In our study nine cases (8.0%) of dislocation were observed after revision THA. We believe that definite repair of short rotators and use of larger heads in our study contributed to better results. Although increase in head size was not associated with reduced rate of dislocation (P=0.905), it might originate from power insufficient to draw definite conclusion on it. We believe that the lower rate of dislocation in our study was attributable to the result. But, the number of dislocations was too small for us to yield reliable results. And in our study, we tried to reduce the dislocation rate by having the femoral offset and the position to the appropriate range. It is generally accepted that the femoral offset and the position of the acetabular cup which includes inclination and anteversion affect the stability and range of motion in THA. The historical guideline for the "safe zone" of component positioning for dislocation avoidance has been defined as a static target of 15°±10° of cup anteversion with 40°±10° of cup inclination28). However, the femoral offset and cup position had no significant association with the dislocation rate. On an average the patients with dislocation were older than those without dislocation, but the difference was not significant. Further evaluations with a larger number of patients are needed to clarify the risk factors of dislocations in revision arthroplasty.
There was no ceramic fracture in this study. Ceramic fractures occur when the implant is exposed to an unexpected high-load pressure, leading to subcritical crack propagation. Avoidance of 28-mm third-generation ceramic with short neck, use of fourth-generation ceramics and acceptable range of implant position may be attributed to low incidence of ceramic fracture in this study.
Compared with other literatures from western countries, the BMI (23.6 kg/m2) of our article is relatively low. Therefore we suppose that the relatively low BMI in this study may have contributed to lower the incidence of ceramic head fracture, decreasing the load pressure to implant, although further study is necessary to confirm this hypothesis. However, the patients in our study underwent revision surgery at relatively lower age (mean 51.6 years) and their activity level is much higher than the patients in other articles. Despite the high activity level of the patients, as there was no ceramic head fracture, our current protocol is to use fourth-generation Delta ceramic heads.
In revision arthroplasty, appropriate placement of the components is most critical in adjusting LLD8). In our study, pre-operative LLD was measured in all patients to guide the surgeon as to how the joint should be reconstructed. Intra-operatively, we had lengthened the neck of the implant if the leg is short, and tried to achieve rotational and axial stability by a press fit technique and firm acetabular bone grafting. As a result, the average of clinical LLD was 0.21 cm (range, 0 to 2 cm) and radiologic LLD was 0.2 cm (range, 0 to 2 cm). To achieve better results, it is necessary to strengthen the host bone by using materials such as allograft-prosthesis composite, tantalum block and cup-cage. However, tantalum augmentation was not available in Korea during the study period.
There are some limitations in this study. First, it was a retrospective study and had a non-comparative design. Second, it was performed in low-BMI patients only. Third, the relatively short-term follow-up period (6.3 years) is noted. We believe that a long-term follow-up period of 10 years or longer is necessary for the assessment of revision THA. However, this study can be considered to be of significance, since most of the complications such as ceramic fractures, dislocations and LLD could be found within two years postoperatively.
Figures and Tables
Fig. 1
Anteroposterior radiographs showing the hip implant with loosening due to collapse of the structural bone graft. The loosening occurred six years after the revision, and the cup showed 3 mm migration, and 16° change in tilting. An allo-femoral head structural bone graft was used to replace the extensive osteolytic lesion, which was type IIIB defect according to the Paprosky's classification of bone defects. One year postoperatively there had been no further complications. (A) Postoperative radiograph after the first revision arthroplasty. (B) Follow-up radiograph 72 months after first revision arthroplasty. (C) Postoperative radiograph after the re-revision.
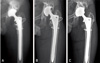
Fig. 2
Anteroposterior radiographs showing the hip implant with dislocation. The dislocation occurred three years after the revision. One year postoperatively there had been no further complications. (A) Postoperative radiograph after the first revision arthroplasty. (B) Follow-up radiograph 36 months after first revision arthroplasty. (C) Postoperative radiograph after the re-revision.
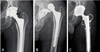
Fig. 3
Kaplan-Meier survival with 95% confidence intervals, with revision for any reason as the endpoint. The survivorships of 5 years and 10 years were 94.5% and 92.9%, respectively.

Table 4
Comparison of Demographic Data between the Patients with Dislocation and the Patients without Dislocation

Values are presented as average (range).
*A Wilcoxon rank sum test was used to estimate the P-value in comparison with age, body mass index (BMI), height, inclination, anteversion and femoral offset. A Fisher's exact test was used to estimate the P-value in comparison with head size, generation of ceramic and gender of patients.
Biolox® Forte and Biolox® Delta manufactured by CeramTec, Plochingen, Germany.
References
1. Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005; 87:1487–1497.

2. Ulrich SD, Seyler TM, Bennett D, et al. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008; 32:597–604.

3. Pospischill M, Knahr K. Cementless total hip arthroplasty using a threaded cup and a rectangular tapered stem. Follow-up for ten to 17 years. J Bone Joint Surg Br. 2005; 87:1210–1215.
4. Zichner L, Lindenfeld T. In-vivo wear of the slide combinations ceramics-polyethylene as opposed to metal-polyethylene. Orthopade. 1997; 26:129–134.
5. Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992; (276):7–18.

6. Brodner W, Bitzan P, Meisinger V, Kaider A, Gottsauner-Wolf F, Kotz R. Serum cobalt levels after metal-on-metal total hip arthroplasty. J Bone Joint Surg Am. 2003; 85-A:2168–2173.

7. Hallab N, Merritt K, Jacobs JJ. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001; 83-A:428–436.

8. Jack CM, Molloy DO, Walter WL, Zicat BA, Walter WK. The use of ceramic-on-ceramic bearings in isolated revision of the acetabular component. Bone Joint J. 2013; 95-B:333–338.

9. Pospischill M, Knahr K. Strategies for head and inlay exchange in revision hip arthroplasty. Int Orthop. 2011; 35:261–265.

10. Paprosky WG, Burnett RS. Assessment and classification of bone stock deficiency in revision total hip arthroplasty. Am J Orthop (Belle Mead NJ). 2002; 31:459–464.
11. Williams VG 2nd, Whiteside LA, White SE, McCarthy DS. Fixation of ultrahigh-molecular-weight polyethylene liners to metal-backed acetabular cups. J Arthroplasty. 1997; 12:25–31.

12. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969; 51:737–755.

13. Jasty M, Harris WH. Salvage total hip reconstruction in patients with major acetabular bone deficiency using structural femoral head allografts. J Bone Joint Surg Br. 1990; 72:63–67.

14. Cadambi A, Engh GA, Dwyer KA, Vinh TN. Osteolysis of the distal femur after total knee arthroplasty. J Arthroplasty. 1994; 9:579–594.

15. revisan C, Nava V, Mattavelli M, Parra CG. Bisphosphonate treatment for osteolysis in total hip arthroplasty. A report of four cases. Clin Cases Miner Bone Metab. 2013; 10:61–64.
16. Tamaki Y, Goto T, Hamada D, et al. Massive femoral osteolysis secondary to loosening of a cemented roughened long stem: a case report. Case Rep Orthop. 2014; 2014:840267.

17. DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976; (121):20–32.

18. Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res. 1990; (257):107–128.

19. Melloh M, Eggli S, Busato A, Roder C. Predictors of early stem loosening after total hip arthroplasty: a case-control study. J Orthop Surg (Hong Kong). 2011; 19:269–273.

20. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978; 60:217–220.

21. Woo RY, Morrey BF. Dislocations after total hip arthroplasty. J Bone Joint Surg Am. 1982; 64:1295–1306.

22. Murphy SB, Simon SR, Kijewski PK, Wilkinson RH, Griscom NT. Femoral anteversion. J Bone Joint Surg Am. 1987; 69:1169–1176.

23. Renkawitz T, Sendtner E, Schuster T, Weber M, Grifka J, Woerner M. Femoral pinless length and offset measurements during computer-assisted, minimally invasive total hip arthroplasty. J Arthroplasty. 2014; 29:1021–1025.

24. Murray DW, Carr AJ, Bulstrode C. Survival analysis of joint replacements. J Bone Joint Surg Br. 1993; 75:697–704.

25. Han CD, Choe WS, Yoo JH. Effect of polyethylene wear on osteolysis in cementless primary total hip arthroplasty: minimal 5-year follow-up study. J Arthroplasty. 1999; 14:714–723.

26. Hooper GJ, Rothwell AG, Stringer M, Frampton C. Revision following cemented and uncemented primary total hip replacement: a seven-year analysis from the New Zealand Joint Registry. J Bone Joint Surg Br. 2009; 91:451–458.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download