Abstract
Although the incidence of sciatic nerve palsy following total hip arthroplasty is low, this complication can cause devastating permanent nerve palsy. The authors experienced a case of sciatic nerve palsy caused by ruptured and contracted external rotator muscles following total hip arthroplasty in a patient suffering from osteonecrosis of the femoral head. We report this unusual case of sciatic nerve palsy with a review of the literature.
According to previous reports, sciatic nerve palsy following total hip arthroplasty (THA) is rare after primary THA 123). Potential causes of sciatic nerve palsy include direct trauma, post-operative pressure by a hematoma, improper traction when using traction tools, excessive leg lengthening, secondary thermal damage due to cement leakage, and damage by trochanteric wire and suture34). Other causes of sciatic nerve palsy include secondary reactions by wear debris, compression from the gluteus maximus tendon, compression by piriformis muscle567). However, in more than 50% of nerve palsy cases the causes remain unknown468).
The authors experienced a case of sciatic nerve palsy caused by contraction of ruptured short external rotator muscles in a patient who underwent THA surgery due to osteonecrosis of the femoral head; since there is a paucity of information regarding such cases, the authors performed nerve exploration and decompression. After 2 years of follow-up, the patient had a favorable clinical outcomes. Therefore, we decided to report this case.
A 43-year-old female patient was diagnosed with osteonecrosis of the right femoral head and therefore was treated with core decompression and replacement of tantalum trabecular metal system (Zimmer, Parsippany, NJ, USA) about 5 months ago in Soonchunhyang University Cheonan Hospital. No improvement was noted and the patient complained of severe pain after primary surgery. Hence, THA was implemented. According to patient's history, she was being treated with antidepressants but nothing unusual was noted. During surgery, the patient was in a lateral decubitus position; trabecular metal prostheses were removed via a skin incision made by the posterolateral approach. Subsequently, the posterior joint capsule was incised in a 'T' shape and then the piriformis muscle, superior musculus gemellus, internal obturator muscle, and inferior musculus gemellus were confirmed and desquamated from the trochanteric insertion site. Desquamated short external rotator muscles were then sutured, followed by drilling of the trochanteric insertion site to restore the short external rotator muscles via the tendon-bone attachment, which was sutured by passing a surgical thread through the drilled holes. Excessive tendon-bone attachment was prevented by checking the hip movement range before and after suturing. A cementless prosthesis with a ceramicceramic articular surface was used as an artificial prosthesis. A 48-mm SPH Delta acetabular cup (Lima Corporate, Udine, Italy), a 32-mm Biolox® Delta articular surface liner (CeramTec, Plochingen, Germany), and a C2 stem (Lima Corporate) were used. Postoperative radiographs indicated that the leg length was increased by approximately 5.0 mm in comparison with that before surgery (Fig. 1). No significant complications (such as postoperative trauma or infection) were noted, while the strength of the lower extremity was found to be Medical Research Council (MRC) grade V. The lower extremity touch sensory was also in the normal range. Three days after surgery, the patient complained of numbness and reduced muscular strength in the right lower limb. Neurologic examination revealed that knee joint extension, ankle joint flexion, and hallux flexus were MRC grade IV, whereas the ankle joint extensor and hallux extensor were grade I. In addition, sensory examination showed a reduction in pain in the dorsum and foot plantar. A medical history review showed that the patient complained of pain at the surgical site when changing posture to the left in her bed in the evening 2 days after surgery. The patient did not report any other problems other than slight pain following surgery, which is considered normal.
The authors suspected peroneal nerve palsy because 1) the patient used an abduction pillow immediately after surgery, 2) sudden symptoms were reported when the patient complained of nerve palsy symptoms, and 3) there was a possibility of peroneal nerve compression around the fibular neck as the leg with palsy symptoms was slightly externally rotated. Initially, we expected that the symptoms would be alleviated by conservative treatment; the patient was discharged from the hospital 2 weeks after surgery. However, there was no symptom improvement during a 4-week follow-up, and electromyography could not be performed due to extreme pain 2 and 4 months after surgery. Electromyography performed 6 and 10 months after surgery indicated 1) right common peroneal nerve lesion approximately at knee level and 2) sciatic nerve lesion (in its dominant common peroneal part) at above-knee level. These lesions were incomplete in nature and were accompanied by axonal degeneration and regeneration. According to neurologic examinations, palsy symptoms were not improved up to 1 year after surgery. After a complete explanation of nerve exploration to the patient, preoperative magnetic resonance imaging (MRI) was performed.
On MRI images, the course of sciatic nerve was compressed by architecture, agglutinated on ruptured short external rotator muscles and their surrounding soft tissues in comparison with the normal side (Fig. 2). Nerve exploration was performed 1 year after THA. We observed that sciatic nerves were penetrating the piriformis muscle, whereas portion of the piriformis muscle and other short external rotator muscles were ruptured from the trochanteric insertion site, shrunken and constricted; the cross sections of these muscles were slightly rolled up and adhered to the surrounding tissues. In addition, ruptured surfaces were compressing the course of the sciatic nerve (Fig. 3A). Short external rotator muscles, including the part of the piriformis muscle that were compressing the nerves, were excised to perform nerve decompression (Fig. 3B). Ten months after surgery, the strength of the ankle joint extensor and hallux extensor was grade III and a reduction in pain sensation was observed. After 13 months, the strength was shown to be grade IV, which was a significant improvement.
Nerve palsy after THA is uncommon but may result in critical complications; multiple studies have addressed this rare complication, and its cause(s), prognosis, and treatments are being extensively investigated.
The incidence rate of nerve palsy after primary THA ranges between 0.09% and 3.7% but is slightly higher in patients with total hip revision, ranging from 2.0% to 7.6%2348). The incidence rate of sciatic nerve palsy after primary THA is even lower, ranging between 0.17% and 1%124).
Patient-related risk factors include 1) the affected area being on the left side, 2) female gender, 3) developmental hip dislocation or hip dysplasia. Surgery-related risk factors include 1) some surgical approaches; 2) total hip revision; 3) excessive nerve tension because of excessive leg lengthening; 4) direct trauma; 5) postoperative pressure by a hematoma; 6) improper traction when using traction tools, located improper sites; 7) cement leakage and thermal damage; 8) secondary reactions by wear debris; and 9) constriction by a trochanteric wire or suture23459). Nevertheless, the causes of more than 50% of nerve palsy cases remain unknown468). In our case, leg lengthening was unlikely to be the cause, because 1) leg lengthening was not significant (approximately 5.0 mm), 2) no palsy symptoms were observed until 3 days after surgery, and 3) symptoms were noticeably improved after nerve exploration. We were unable to find any evidence for direct pressure caused by a hematoma at the surgery site, such as foreign sensation or swelling. Nerve exploration also did not indicate direct compression by a hematoma. The presence or absence of a hematoma, which may potentially form right after the rupture of short external rotator muscles, was not certain as MRI and computed tomography were not performed immediately after the occurrence of nerve palsy. According to Hurd et al6), more than half of the cases of unexplained palsy might be due to sciatic nerve palsy caused by pressure between femoral attachment sites of the ischial tuberosity and gluteus maximus, as suggested by assessment using MRI. In our case, MRI images showed architecture attached to the short external rotator muscles (rather than compression of the sciatic nerves), which modified the course of the nerves, thus indirectly causing compression. Sosna et al.7) reported a case of sciatic nerve palsy caused by compression of anatomically malformed sciatic nerves by the piriformis muscle that underwent tenotomy after primary THA. Our case is somewhat similar to these observations, because nerve exploration indicated that part of sciatic nerves were penetrating the piriformis muscle; in addition, some of the ruptured piriform muscle and shrunken short external rotator muscles were directly compressing the sciatic nerves.
In general, nerve palsy does not have a favorable prognosis1234). It was reported that approximately 35% of nerve palsy cases become permanent, while 36% of palsy patients with complete motor paralysis recovered after 21.1 months in average13). In the study by Oldenburg and Müller2), only one third of nerve palsy patients were able to fully recover their functionality. In another study with a total of 3,126 patients, Schmalzried et al.4) found that prognosis of nerve recovery may be associated with the degree of nerve damage; therefore, it is difficult to expect satisfactory recovery for patients with serious paresthesia. Further, sciatic nerve palsy tends to be found in peroneal components rather than in tibial component and is more serious in most cases234).
Kyriacou et al.9) demonstrated that a significant reduction in pain may be achieved by nerve exploration and neurolysis in patients with sciatic nerve palsy after THA. Furthermore, there was no significant association between the timing of sciatic nerve exploration and the degree of pain reduction; therefore, it would be still beneficial to perform exploration until up to 40 months after surgery. Unwin and Scott10) suggested that the presence of pain in the affected nerves might be the most important factor to consider in order to determine whether nerve exploration is necessary in patients with acute palsy. If a patient has nerve palsy accompanied by pain, nerve exploration is recommended. Weber et al.8) reported that peroneal nerve palsy after THA might be due to direct damage of the sciatic nerves at surgical sites rather than at the knee level. Similarly, Schmalzried et al.4) reported that THA-related nerve injuries are observed around the hip. In the present case, exploration was delayed because of somewhat inconsistent results of electromyographic analyses that were performed 6 and 10 months after THA; in these analyses, lesions of the peroneal nerves and sciatic nerves were shown, respectively. These results suggested either completely different lesions at different sites or examination errors. Soon after surgery, the patient did not complain of any noticeable pain other than general pain at the surgical site; furthermore, nerve palsy was initially found around the knee, potentially owing to the patient's leg posture and the use of an abduction pillow, which prompted us to implement conservative treatment. Although surgical treatment was successful in improving symptoms in this case, we were unable to perform nerve exploration and neurolysis at an early stage due to the 12-month conservative treatment, which failed to achieve favorable clinical outcomes. Consequently, this resulted in an undesirably long recovery period.
On the basis of this experience, the authors would like to suggest the necessity of a careful follow-up after THA regarding sciatic nerve palsy potentially caused by ruptured short external rotator muscles. Early nerve exploration and neurolysis might be warranted if unexplained palsy is observed.
Figures and Tables
Fig. 1
(A) Preoperative radiograph. (B) The immediate postoperative radiograph shows good positioning of the prosthesis.
Fig. 2
(A) Coronal T2-weighted image of the right hip shows the loss of fascicular architecture and thickened sciatic nerve, which was compressed laterally (white arrow). (B) Coronal T2-weighted image of the left hip shows the normal course of the sciatic nerve. (C) Axial T1-weighted image shows that the sciatic nerve, which adhered to the short rotator muscles and soft tissues, was compressed posteriorly (black arrow).
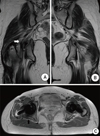
Fig. 3
(A) A close photograph shows that the sciatic nerve (1) was severely tethered and compressed by the ruptured and contracted part of the piriformis muscle, short extensor rotator muscles, and surrounding soft tissue (2). Black arrow indicates the line of ruptured short external rotator muscles. (B) After decompression, the sciatic nerve was freed.

References
1. Farrell CM, Springer BD, Haidukewych GJ, Morrey BF. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am. 2005; 87:2619–2625.

2. Oldenburg M, Müller RT. The frequency, prognosis and significance of nerve injuries in total hip arthroplasty. Int Orthop. 1997; 21:1–3.

3. Nercessian OA, Macaulay W, Stinchfield FE. Peripheral neuropathies following total hip arthroplasty. J Arthroplasty. 1994; 9:645–651.

4. Schmalzried TP, Amstutz HC, Dorey FJ. Nerve palsy associated with total hip replacement. Risk factors and prognosis. J Bone Joint Surg Am. 1991; 73:1074–1080.

5. Crawford JR, Van Rensburg L, Marx C. Compression of the sciatic nerve by wear debris following total hip replacement: a report of three cases. J Bone Joint Surg Br. 2003; 85:1178–1180.

6. Hurd JL, Potter HG, Dua V, Ranawat CS. Sciatic nerve palsy after primary total hip arthroplasty: a new perspective. J Arthroplasty. 2006; 21:796–802.
7. Sosna A, Pokorny D, Jahoda D. Sciatic nerve palsy after total hip replacement. J Bone Joint Surg Br. 2005; 87:1140–1141.

8. Weber ER, Daube JR, Coventry MB. Peripheral neuropathies associated with total hip arthroplasty. J Bone Joint Surg Am. 1976; 58:66–69.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download