INTRODUCTION
Total hip arthroplasty (THA) is extensively performed on a considerable number of patients with advanced stages of osteonecrosis of femoral head (ONFH) in Korea. Core decompression, multiple drilling, different types of bone grafting and osteotomy are interventional options for early stages of ONFH to preserve the femoral head and avoid arthroplasty. However, ONFH is usually detected at advanced stages with a severely collapsed femoral head and acetabular osteoarthritis (Ficat stages III and IV). Since joint preserving surgery is not effective in these patients, arthroplasty becomes the primary treatment option1). Important hip arthroplasty techniques are hemiresurfacing arthroplasty, bipolar hemiarthroplasty, total hip arthroplasty, total resurfacing arthroplasty2).
ONFH occurs primarily in young and active men in their 20s-40s. Different factors are described to be involved in the pathophysiology of ONFH3,4). THA has the most favorable outcome for ONFH and is continuously improved by advances in development of bearing surfaces, components and types of prostheses, and surgical techniques. Ceramic-on-ceramic bearings have the best outcome in terms of low wear rate, remarkable wear resistance, hydrophilic property and high biocompatibility. Important risks associated with this bearing are ceramic fracture and squeaking. Due to postoperative outcomes and long-term performance, arthroplasty is the treatment of choice in patients with ONFH, particularly in younger patients3,4). Younger patients expect to regain ability to perform daily life activities without any limitation. Limitations in the range of motion, fear of dislocation and pain in the inguinal and femoral regions are frequently experienced following arthroplasty. Long-term complications include osteolysis caused by wear debris between bearing surfaces, aseptic loosening. Clinical outcomes of THA is inferior in younger patients participating in high-impact activities in comparison to THA in elderly patients with degenerative arthritis5,6,7). Although the 10-year survival rate of bipolar hemiarthroplasty is remarkably high at about 90% for femoral neck fracture8), adverse reactions including inguinal pain and prosthesis movement, and, complications such as osteolysis are reported in a considerable number of patients receiving this treatment9). The advatage of hemiresurfacing arthroplasty is conservation of femoral bone stock by solely resurfacing the lesioned segment with a metal bearing surface, but severe inguinal pain remains a challenge for this method.
In metal-on-metal hip resurfacing, the damaged surfaces of the femoral head and the acetabulum are removed and resurfaced with metal-on-metal bearing surfaces. This procedure preserves the proximal bone stock around the femur and is highly safe in terms of dislocation because a better range of motion is provided using a large-diameter head that enables sport activities. Metal-on-metal articulations are less prone to wear and loosening than conventional metal-on-polyethylene articulations. The disadvantages of this technique include survival of the remaining femoral head following resurfacing arthroplasty, progression to osteonecrosis, femoral neck fracture, tissue reactions from metal debris. This paper aims to introduce different hip replacement techniques for ONFH and elaborate characteristics of each technique.
MAIN BODY
1. Hemiresurfacing Arthroplasty
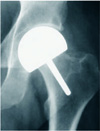
This procedure involves the replacement of femoral head surface only using appropriately sized prosthetic head after removal of the necrotic lesions from the femoral head with cement fixation (Fig. 1). Since the original biomechanics of the hip joint is preserved, activity levels can be sustained in young patients with a low rate of dislocation. Bone stock and marrow cavity of the femur is sufficiently preserved by only removing the cartilage and bone stock of the femoral head. Moreover, THA can be easily performed if prompted.
Hemiresurfacing arthroplasty can induce severe inguinal pain in the presence of preexisting damage of the acetabular cartilage. In a comparative study by Mont et al.10) conducted between two groups who underwent hemiresurfacing arthroplasty and traditional THA, 60% of patients of hemiresurfacing arthroplasty group were able to perform high-impact exercise while 20% of patients had severe groin pain in the 7-year follow-up. Survival rates were similar being 90% in hemiresurfacing arthroplasty group and at 93% in THA group. According to Beaulé et al.11), the longevity of hemiresurfacing arthroplasty is 79% at 5 years, 59% at 10 years and 45% at 15 years of follow-up. Cuckler et al.12) followed 59 patients for an average of 4.5 years after hemiresurfacing arthroplasty and found that16 patients (32%) received revision THA due to severe pain in the inguinal area.
Theoretically, hemiresurfacing arthroplasty is a less invasive surgical procedure for young patients with advanced ONFH that cannot be managed with joint preserving surgery. Hemiresurfacing arthroplasty can be recommended to young patients with ONFH at Ficat and Arlet stage III, patients with ONFH for lesions involving more than 30% of the femoral head, patients with femoral head surface collapse of at least 2 mm and those without acetabular cartilage damage13,14). The major drawback of this technique is postoperative groin pain after which this technique is rarely used.
2. Bipolar Hemiarthroplasty
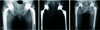
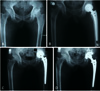
The indications of bipolar hemiarthroplasty are same as hemiresurfacing arthroplasty. Bipolar hemiarthroplasty is designed to reduce the shearing stress that is imposed on the acetabulum in hemiresurfacing arthroplasty by providing bipolar movement between the inner bearing and femoral head. Grevitt and Spencer15) followed 22 ONFH patients who underwent bipolar hemiarthroplasty with cemented femoral components with a mean age of 40 years. Favorable outcomes were observed without severe erosion of articular cartilage and early loosening of femoral stem. Chan and Shih16) found no difference in clinical scores, groin pain, thigh pain, osteolysis, dislocation and revision rates between two groups (28 patients receiving cemetless THA and 28 others receiving cemetless bipolar hemiarthroplasty) followed for an average of 6.4 years. Similar to hemiresurfacing arthroplasty, bipolar hemiarthroplasty might also require revision surgery due to groin pain arising from friction between cartilage and metal head, acetabular cartilage erosion, acetabular defect (Fig. 2), and protrusio acetabuli (Fig. 3). There is also a risk of osteolysis due to polyethylene wear debris. Lachiewicz and Desman17) detected the wear of the acetabular cartilage and upward displacement of the femoral head in 14 out of 31 cases followed for an average of 4.6 years. Ito et al.8) reported groin pain in 42% and revision rate in 25% in an average of 11.4 years follow-up. Therefore, bipolar hemiarthroplasty is mainly performed on elderly patients with femoral head fracture without acetabular lesions. It is gradually falling out of favor in patients with ONFH.
3. Total Hip Arthroplasty
THA is the most common surgical procedure for the treatment of ONFH.
THA is particularly effective in patients at advanced stages of the disease associated with severely collapsed femoral head and acetabular osteoarthritis (Ficat stage III and IV), and after the failure of joint preserving surgery, which include core decompression, osteotomy and resurfacing arthroplasty. According to long-term follow-up findings, THA has a low success rate in young patients with ONFH compared to those with other causes such as osteoarthritis and rheumatoid arthritis7). Higher rates of failure and complication is shown in ONFH caused by steroid use or associated with comorbid disorders (sickle cell anemia, systemic lupus erythematosus, kidney transplant) compared to idiopathic ONFH6,18,19). Acurio and Friedman18) conducted THA on 25 patients with ONFH caused by sickle cell disease and found that revision surgery was required in 14 cases (40%) and observed complications in 49%. Lieberman et al.19) reported a high failure rate of THA in 81% of patients undergoing renal transplant. Furthermore, Brinker et al.5) found a high failure rate and complication in patients with systemic lupus erythematosus.
Park et al.20) found no significant difference between osteotomy and non-osteotomy groups in terms of clinical outcomes following primary THA on ONFH. Lee et al.21) observed non-significant differences in postoperative complications, clinical outcomes and implant stability, although potential risks such as prolonged operation time, massive bleeding, and incorrect implant insertion were higher in osteotomy group.
Along with recent developments in the design of bearing surfaces, the new designs and fixation methods of femoral prosthesis, improved component materials and cement techniques have led to successful results in THA performed on ONFH and other diseases22).
In cemented THA, improved outcomes were achieved with recent advancements in cementing techniques. With the use of second-generation cementing techniques, the success rate was 86% in 28 cases followed up for 92 months in a study by Kantor et al.22), and 96% in 123 cases followed up for an average of 54 months in a study by Garino and Steinberg23) In uncemented THA, high success rate was shown with the advancements in fixation method and design of prosthetic implants. In a 64-month follow-up study conducted by Phillips et al.24) loosening of femoral components was observed in 1 out of 20 cases who received THA using an uncemented porous-coated anatomic hip, but there was no revision surgery. Femoral components of hip prostheses need to be chosen carefully according to the shape of femur, cortical thickness, bone strength, and the condition and quality of cross section of cancellous bone regardless of patient's age.
A success rate of over 90% has been observed over half a century with no complications (such as toxicity) with existing polyethylene-metal surfaces25). Severe wear on surfaces after hip arthroplasty causing osteolysis, implant loosening and hip joint dislocation has been identified as a major cause for revision surgery26). Failure rate was 48% in 29 cases with cemented THA followed up for 84 months in Saito et al.'s study7), and 39% in Cornell et al.'s study27). Consequently, extension of the longevity of bearing surfaces is a focus of research.
Conventional polyethylene acetabular liners are treated with 2.5-4.0 Mrads gamma radiation. Free radicals are created during radiation and result in oxidation. Oxidation weakens the strength of the polyethylene and reduces resistance to wear. To overcome these problems, highly cross-linked polyethylene (HXLPE) was developed by enhancing the cross-linking of polyethylene particles using 5 to 10 Mrads gamma radiation or electron beam radiation. Unreacted free radicals remaining after cross-linking are eliminated with remelting of polymers at 125-135℃ melting points and annealing temperature slightly below the melting temperature. Although remelting method completely eliminates residual free radicals, it weakens crystallinity and stiffness of polyethylene. In annealing method, the mechanical properties are well maintained, but residual free radicals are incompletely eliminated28,29). The degree of polymer cross-linking is proportional to the radiation dose, whereas the degree of cross-linking is inversely proportional to the wear rate. When cross-linking increases, the physical properties of polyethylene weakens, which include ultimate tensile strength, ductility, toughness and fatigue strength. To resolve these problems, new manufacturing technologies for HXLPE have been developed. In the first technology, antioxidant vitamin E is used to stabilize HXPE by blending liquid vitamin E with ultra-high molecular weight polyethylene resin before compression molding or diffusion of vitamin E into already consolidated and irradiated polyethylene to improve oxidation resistance. In the second technology, gamma irradiation is used three times at the dose of 3 Mrad to reduce oxidation caused by free radicals that were produced from irradiation (X3™; Stryker Orthopaedics, Mahwah, NJ, USA). Favorable outcome are anticipated with these new polyethylenes.
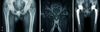
Newly developed ceramic-on-ceramic bearing surfaces show favorable mid- to long-term clinical outcomes (Fig. 4). During early stages of development, acetabular loosening was frequently observed because the ceramic cup was directly fixed or cemented into the acetabulum in alumina-alumina articulation, and a high fracture rate was seen in ceramic components with low purity and large ceramic particles. Along with recent advances in ceramic production, ceramic implants have currently high purity and density with favorable mechanical characteristics including hardness, bending strength and burst strength. Nich et al.30) and Fye et al.31) reported almost no osteolysis resulting from high wear resistance on ceramic-on-ceramic surfaces, and, high success rate in mid- and long-term follow-up.
The benefits of THA with ceramic surfaces are outstanding wear resistance, insignificant biological responses to wear debris and low incidence of osteolysis. For these reasons, this method has recently emerged as the best option for active young patients. However, the risk of ceramic fracture and squeaking remains. The risk factors for ceramic component fracture include sandwich structures32), short neck and ceramic head33), and the possible causes of squeaking include implant design34), stripe wear due to edgeloading and loads and friction on the surface corners imposed by the high inclination of the acetabular cup35) which is controversial. To prevent these complications, precise positioning of the acetabular component and proper inclination of the acetabular cup is required. Consistent effort needs to be made to minimize fractures by instructing patients to avoid any impact activity during postoperative rehabilitation.
4. Total Resurfacing Arthroplasty
Total resurfacing arthroplasty originated from mold arthroplasty that was first introduced by Smith-Peterson et al.36,37) and attempted with different materials and fixation methods. The use of this method had been almost discontinued since mid-1970s due to serious wear and high failure rate caused by loosening. In the late 1990s, this procedure was developed as a surgical alternative to total hip replacement for active young patients by McMinn et al.38)
In hip resurfacing, the femoral head is not removed, but is instead trimmed, and the damaged parts within the socket is removed and replaced with a metal shell. Unlike traditional THA, this method can preserve biomechanical properties including the length of the lower extremities, offset, femoral anteversion, and the normal length of the adductor lever. Although the technique is still controversial, postoperative joint stability and extended range of motion enable patients to return to daily life and sport activities with the increased jump distance of larger heads. Moreover, femoral components can be more easily replaced when the joint needs THA (Fig. 6). According to Daniel et al.39), 92% of one hip resurfacing and 82% of both hip resurfacing patients were able to perform sport activities among 446 patients who received hip resurfacing. Since large femoral head diameter, similar to the size of the patient's original head, is used for metal-metal surfaces, jumping distance is required before dislocation increases (Fig. 5). Suction fit occurs in hip joints, in which, interfacial adhesive force increases between the head and the cup in the presence of lubricant. Consequently, a considerably low chance of hip dislocation is observed at less than 1% regardless of the surgical approach39,40,41).
Although metal-on-metal bearing surfaces have slightly higher wear rate and osteolysis than ceramic-on-ceramic surfaces in total resurfacing arthroplasty, they have slightly lower wear rate and osteolysis compare with metal-on-polyethylene surfaces. Higuchi et al.42) and Jantsch et al.43) observed considerably low wear rate and osteolysis incidence at revision surgery followed by McKee-Farrar THA. Additionally, the second-generation metal-on-metal implant Metasul, first introduced by Müller, Weber, Zweimüller and Spotorno, also exhibited a low wear rate of 2-5µm and low incidence of osteolysis44). Since nanometer-sized metal wear particles are less subject to phagocytosis by macrophages compared with polyethylene or cement particles, the incidence of local osteolysis is low. Although the occurrence of femoral neck narrowing and various forms of osteolysis lesions has been reported, the overall wear rate and osteolysis incidence were lower than those of metal-on-polyethylene surfaces in THA.
Infection following total resurfacing arthroplasty occurred in 1 out of 200 cases in a study by Amstutz et al.45), 0 out of 426 cases in a study by Wagner46), and 0 out of 446 cases in a study by Daniel et al.39) which stem from substantially small dead space around the joint and small prosthesis size. Infection rate was low compared to that of THA.
The clinical outcomes of total resurfacing arthroplasty are still controversial. Grubl et al.47) reported that total resurfacing arthroplasty was successful in 94% of 36 ONFH patients followed up for an average of 42 months. In a comparative study by Mont et al.48), metal-on-metal resurfacing was performed on 42 osteoarthritis and ONFH cases and patients were followed up for an average of 41 months. Success rates were comparable for both groups at 98% and 93%. According to Beaulé et al.49) success rate was 95% in 56 cases followed up for an average of 60 months. On the other hand, recent studies reported unfavorable results in total resurfacing arthroplasty. According to a 9-year follow-up study conducted by de Steiger et al.50), revision rate for total resurfacing arthroplasty was 7.2%, higher than that of traditional THA at 5.4%. The leading causes of early failure were suggested to be femoral neck fracture, avascular necrosis, and component loosening. Since high osteolysis and pseudotumor incidences were observed in total resurfacing arthroplasty using ASRTM (Depuy Orthopaedics, Warsaw, IN, USA), the use of ASR components is currently stopped51,52). Risk factors for the failure of total resurfacing arthroplasty are thought to be acetabular prosthesis design, loads on the surface corners imposed by improper placement of the acetabular components, and excessive increases in serum metal ion levels caused by excess wear.
It is still not known if serum metal ion levels increased by metal debris particles affect the human body, if there is any potential risk of ONFH caused by medial femoral circumflex artery obstruction, and if there is an increased risk of femoral neck fracture and femoral component loosening. Since serum and urine metal ion levels increase due to debris particles from metal joints in total resurfacing arthroplasty40,43), a study was conducted and showed induction of tumors in rats by the injection of wear debris from cobalt-chrome alloy implants52). Another study suggested the development of cancer after McKee-Farrar THA46). However, tissue reaction to metal debris from metal-on-metal joints is more a granulomatous inflammatory reaction compare to the reaction caused of polyethylene wear debris. The incidence of osteolysis is lower around the implants because fewer number and smaller size of metal wear particles facilitate the movement of wear particles from joints26), and the toxicity in surrounding tissues has not been proven53). According to a recent study by Delaunay et al.53), no evidence was found to support that increased serum metal ion levels and accumulated metal debris incur toxicity and carcinogenesis after more than four decades of metal-on-metal joint use. There are still controversial issues requiring further investigation including delayed hypersensitivity of increased serum metal ion concentrations, osteolysis, transfer of metal ions to fetus through the placenta. Although the occurrence of pseudotumor has been dealt with in previous studies54,55) on conventional THA, a substantially higher incidence of pseudotumor formation on metal-on-metal surfaces mandates careful consideration in patient selection and surgical techniques.
When hip resurfacing is performed on active young patients with ONFH using meticulous surgical techniques and strict patient selection, it preserves the joint acceptably, improves limitations of traditional THA, and becomes an ideal artificial joint securing high-impact activities. Additional long-term follow-up studies are essential to further investigate an increase in serum metal ion levels observed with metal-on-metal joints, metal hypersensitivity, risk of carcinogenesis, and potential impact on the human body.
CONCLUSION
The treatment for advanced ONFH aims to decrease pain and improve postoperative function and the quality of life; but the selection of surgical methods remains controversial. For appropriate surgical decision making, patient's age, physical activity level, underlying diseases, pathologic stage and the degree of necrosis should be taken into account. Hemiresurfacing or total resurfacing arthroplasty can be considered for active young men with small sized necrosis. THA is more feasible for physically inactive patients with extensive necrosis and acetabular invasion. Newly developed bearing surfaces (ceramic-on-ceramic, metal-on-metal, metal-on-highly cross-linked polyethylene) and recent advances in surgical techniques are expected to lead to desirable outcomes. Satisfactory results can be achieved in young patients with advanced ONFH when the surgery is performed carefully by understanding the characteristics of each bearing articulation and suitable indication.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download