Abstract
This is the first case report on the effects of teriparatide on the course of healing of an intertrochanteric hip fracture with unusually excessive callus formation even after discontinuation of treatment in an elderly woman. This case highlights the long-term effects of parathyroid hormone, even after administration of short-term, intermittent dosages for healing of osteoporotic fracture.
Parathyroid hormone (PTH) has long been shown to have anabolic and catabolic effects on the skeleton. At low intermittent doses, PTH administration leads to increased bone formation in rats and humans. Currently, various human trials have demonstrated the anabolic effects of teriparatide (recombinant human PTH [1-34]) in both women and men with osteoporosis and that treatment causes a substantial decline in fracture frequency. Correspondingly, external callus volume, callus mineral content, and callus dimension have been shown to increase significantly from 8-16 weeks of healing in teriparatide-treated patients1). We have analyzed the effects of teriparatide (Forteo; Eli Lilly and Company, Indianapolis, IN, USA) on fracture healing in an 85-year-old woman with osteoporosis who sustained a comminuted intertrochanteric fracture (OTA/AO 31-A2.2), and found that teriparatide significantly accelerated the amount of callus formation around the fracture site even after completion of teriparatide treatment, which is an unusual phenomenon during a normal fracture healing process.
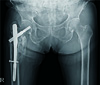
We present the case of an 85-year-old woman admitted to our hospital 3 years ago with a history of falling at home one day prior to admission because of slipping on the floor. The patient had a history of pain, swelling, and deformity of the right hip, and no history of injury to any other body part. She was diagnosed with an osteoporotic, comminuted intertrochanteric fracture (Fig. 1). After medical fitness, the patient underwent closed reduction and intramedullary nailing with a cephalomedullary nail-PFNA (Synthes, West Chester, PA, USA) (Fig. 2). Surgery was uneventful. She gave informed consent for therapy with teriparatide after being informed about the possible benefits, side effects, and risks. In the 3 postoperative days, the patient was started on a daily 20µg subcutaneous intra-abdominal injection of teriparatide for up to 3 months. Before starting PTH treatment, the patient's detailed medical history was recorded and physical and laboratory evaluations were conducted. It was confirmed that she had no prior treatment with any form of postmenopausal antiosteoporotic medication, no history of bone or other systemic metabolic disorder such as active liver disease or clinical jaundice; or symptomatic nephro- or urolithiasis, no history of malignant neoplasm, and no prior risk of radiotherapy exposure. She had also never had renal dialysis, obesity, or anemia. And she had not taken steroids or other immunosuppressive agents.
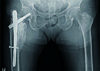
The patient also was given daily supplementation of 1,000 mg of calcium and 800 IU of vitamin D3 in the postoperative period. Postoperatively, serial blood monitoring for serum calcium, phosphorus, alkaline phosphatase, vitamin D, and uric acid levels was performed, and normal limits were confirmed during the entire course of teriparatide treatment. We, however, did not perform routine measurements of other biochemical markers of bone turnover (N-propeptide of type 1 collagen, urinary N-telopeptide, urine deoxypyridinoline, serum C-terminal telopeptide) because the tests of bone turnover markers were not recommended to monitor the response to treatment with teriparatide2). Fracture healing was assessed on serial examination of the pelvis and both hips on anteroposterior and lateral radiography as well as on computed tomography at 4, 8, and 12 weeks until 1-year postoperatively (Fig. 3, 4). At 6 postoperative months, the patient's radiographs showed abnormal excessive callus formation around the intertrochanteric fracture site, which is an unusual presentation without any treatments (Fig. 5). Moreover, she was in the absence of clinical features such as pain, swelling or loss of rotational movement.
To our knowledge, this is the first case report on the effects of teriparatide on the course of healing of an intertrochanteric hip fracture with unusual excessive callus formation in an elderly woman with osteoporosis in the absence of Paget's disease of the bone, osteosarcoma of the bone, and history of radiation.
Based on the large number of case studies, it is evident that teriparatide treatment can enhance normal fracture healing. Correspondingly, both callus volume and callus mineral content are augmented in teriparatide-treated animals3). Animal experiments have also shown that PTH increases new bone formation around implants. PTH treatment augments bone mass density in implanted titanium bone chambers in a dose-dependent manner4).
The exact mechanisms by which teriparatide stimulates bone healing in various animal and human studies has not been clarified. However, teriparatide should be considered as treatment for postmenopausal women with severe osteoporosis and the management of individuals at particularly high risk for fractures. Therefore, we continuously used teriparatide for only 3 months even though we do not know exactly whether excessive callus may be associated with the duration of using teriparatide or not because teriparatide therapy is not recommended for more than 2 years based on the induction of osteosarcoma in a rat model of carcinogenicity5).
Currently, various human trials have demonstrated the anabolic effects of teriparatide treatment in both women and men with osteoporosis, and that treatment causes a substantial decline in fracture frequency. A recent study on teriparatide (PTH [1-34]) regarding the acceleration of fracture healing was conducted in 102 postmenopausal women with distal radial fracture. The limitations of this human study are that it focused on callus volume only up to 5 weeks, and there was no long-term evaluation of the subsequent effects of teriparatide on the distal radial fracture6). Another study, by Peichl et al.7), showed that teriparatide accelerated fracture healing in the pubic bones of elderly women with osteoporosis. Their study was limited, however, because it focused on the effects of teriparatide on fracture healing only until radiographic evidence of cortical bridging at the fracture site, and there were no long-term effects on callus volume or quality after discontinuation of teriparatide treatment.
The findings of this case report, in contrast with prior studies, explore another important impact of the clinical utilization of this drug for human fracture healing. Although previous experimental human studies have demonstrated the acceleration of fracture healing, none have assured the safety of the long-term effects of teriparatide, such as an unusual, uncontrolled, excessive callus formation, even after radiographic healing of the fracture.
In conclusion, this case report provides information regarding monitoring of the long-term effects of teriparatide on fracture healing, especially in osteoporotic fractures and those around the hip, which might help create a risk-benefit comparison over a long-term duration.
Figures and Tables
Fig. 1
Preoperative anteroposterior radiograph of the pelvis, hips, and femurs, showing osteoporotic, 4-part comminuted, displaced intertrochanteric fracture of the right hip of AO/OTA classification 31-A2.3 with varus external rotation deformity of the proximal femur.
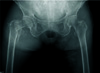
Fig. 2
Immediate postoperative radiograph of the pelvis, hips, and femurs, showing somewhat reduced, stable fracture fixation with proximal femur intramedullary interlock nail with wound drain and urinary catheter in situ.
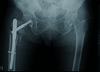
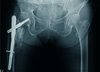
Fig. 3
Three-month postoperative radiograph of the pelvis, hips, and femurs, showing well-united fracture site with sufficient periosteal callus formation and stable intramedullary interlock nail in situ.
References
1. Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R. Anabolic actions of parathyroid hormone on bone. Endocr Rev. 1993; 14:690–709.

2. Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005; 26:688–703.

3. Andreassen TT, Ejersted C, Oxlund H. Intermittent parathyroid hormone (1-34) treatment increases callus formation and mechanical strength of healing rat fractures. J Bone Miner Res. 1999; 14:960–968.

4. Skripitz R, Andreassen TT, Aspenberg P. Parathyroid hormone (1-34) increases the density of rat cancellous bone in a bone chamber. A dose-response study. J Bone Joint Surg Br. 2000; 82:138–141.
5. Vahle JL, Sato M, Long GG, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002; 30:312–321.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download