Abstract
Purpose
Deep infection after hip and knee arthroplasty is a serious complication and is difficult to treat due to its toxicity. The aims of our study were to find out the differences of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-sensitive Staphylococcus aureus (MSSA) infection after hip and knee arthroplasty focusing on clinical course and laboratory findings.
Materials and Methods
We retrospectively reviewed 61 staphylococcal infection cases after hip and knee arthroplasty (MSSA in 25 patients, MRSA in 36 patients). Vital signs, laboratory tests, microbiology and clinical courses were analyzed. The average follow-up period was 3.8 years (range, 2 to 10.1 years).
Results
At initial visit, MRSA group showed significant higher erythrocyte sedimentation rate, C-reactive protein (CRP) and neutrophil percentage. The average duration for the normalization of CRP was longer in MRSA group (MRSA: 36.7±25.1 days, MSSA: 24.7±13.6 days; P=0.008). The mean interval between staging operation was longer in MRSA group (MRSA: mean 8.7 weeks [range, 6.4 to 21.4 weeks], MSSA: mean 6.8 weeks [range, 6 to 13.1 weeks]; P=0.012). MRSA group (13.9%) revealed higher recurrence rate than MSSA group (4%). Two patients (5.6%) from MRSA group expired by sepsis. One limb amputation (2.7%) was carried out in MRSA group.
Periprosthetic joint infection (PJI) after hip and knee arthroplasty is one of the most serious complications. Despite routine use of prophylactic antibiotics for surgery, dental procedures, the incidence of PJI remains 0.3-1.7% for total knee arthroplasty (TKA) and 0.8-1.9% of total hip arthroplasty (THA)1,2,3). It is known that prognosis shows large differences depending upon the causative bacteria or treatment methods4). Therefore, it is very important to perform appropriate treatment after precise diagnosis is made. It is known that methicillin-sensitive Staphylococcus aureus (MSSA) is the most common bacteria, but lately as the frequent use of broad-spectrum preventive antibiotics is increasing, methicillin-resistant Staphylococcus aureus (MRSA) infection within hospital or social infection is increasing exponentially. Recently some reports said that there were significant increases in the rates of primary MRSA, as the overall proportion of PJIs from MRSA more than doubled in the latter half from 1998 to 20115,6). Many studies are reporting that if deep infection is caused by MRSA, then it has higher toxicity and damage than those by MSSA. And so MRSA infections come to be more complications, and higher mortality rate7,8,9,10,11,12). Parvizi et al.13) also found out that MRSA-related surgical site infections nearly doubled the length of hospital stay compared with non-MRSA infections. But there was a less study about the clinical course of MRSA PJI in Korea.
The aims of our study were to examine the difference of MRSA and MSSA infection after hip and knee arthroplasty focusing on laboratory results and clinical course.
This study designed as retrospective study and was done in one institution. The present study was approved by our local institutional review boards and all patients provided informed consent. Among 142 patients who were diagnosed as PJI of hip and knee from 1998 to 2011 (including the 108 cases transferred from other hospital, PJI from our hospital: 34 cases, our institution's incidence of PJI-THA: 0.65%, TKA: 0.92%), we selected 66 patients who were proved to have staphylococcal infection by aspiration or wound culture. All of these patients were treated with two-stage revision operation because of chronic infection stage14). We retrospectively analyzed 61 patients (29 knee joints, 32 hip joints). Five patients who had accompanying infection (3 respiratory system infection, 2 urinary tract infection) which could be confusing factor to initial laboratory finding were excluded. MRSA group was consisted of 36 patients and MSSA group had 25 patients. In MRSA group, 20 cases were infected THA and 16 cases were infected TKA. In MSSA group, 12 cases were THA and 13 cases were TKA (Table 1). Infection diagnosis criteria follows as the proposed criteria by the Musculoskeletal Infection Society15): (1) There is a sinus tract communicating with the prosthesis or (2) A pathogen is isolated by culture from at least two separate tissue or fluid samples obtained from the affected prosthetic joint; or (3) Four of the following six criteria exist. Elevated serum erythrocyte sedimentation rate (ESR) and serum Creactive protein (CRP) concentration, elevated synovial white blood cell (WBC) count, elevated synovial neutrophil percentage (PMN%), presence of purulence in the affected joint, isolation of a microorganism in one culture of periprosthetic tissue or fluid, or greater than five neutrophils per high-power field (HPF) in 5 HPF observed from histologic analysis of periprosthetic tissue at ×400 magnification. There were 48 cases which satisfied criteria (2) and 3 cases satisfied criteria (1). All of cases satisfied with criteria (3). We recorded previous MRSA infection history, vital sign (heart rate, body temperature), neutrophil differential rate (%), absolute neutrophil count (ANC), WBC count, hematocrit (%), platelet count, ESR, and CRP of patients when they were diagnosed as PJI. We examined symptom duration, past medical history, antibiotics usage and weight bearing possibility at presentation, and previous MRSA infection history. We also examined the duration for normalization of ESR and CRP, the duration from the first stage mobile articulating spacer (we used prosthesis of antibiotic-loaded acrylic cement [PROSTALAC]) insertion operation to the second stage reimplantation operation. Additionally we evaluated a recurrence rate for minimal 2 year follow up period (mean 3.8 years, range 2 to 10.1 years).
For the 61 patients, we conducted the two-stage revision arthroplasty using mobile articulating spacer by three surgeon using same technique. For mobile articulating spacer, we inserted mixing cement containing gentamycin with vancomycin 4 g16,17,18)(Fig. 1, 2).We conducted bacteria culture test and antibiotic sensitivity test with clinical specimen that collected, while performing joint arthrocentesis or the first mobile articulating spacer insertion operation. We used the first generation cephalosporin antibiotics empirically until the result came out19), and changed antibiotics to vancomycin 1 g per 12 hours only for the group in which MRSA was cultured. We performed the second stage revision arthroplasty when after minimum 6 weeks intravenous antibiotics treatment period, infection indicator was improved and normalized with ESR under 22 mm/hr, CRP under 0.3 mg/dl, and WBC under 10,000/µL. And then polymorphonuclear leukocyte (PML) was detected less than 5 on HPF by frozen section examination15,20). If there were more than 5 on HPF, we did redebridement procedure and kept intravenous antibiotics until infection indicator normalized again.
We compared MSSA group and MRSA group for each variable through Student t-test. We conducted Mann-whitney U-test for all the variables that didn't represent normal distribution. We evaluated with mean±standard deviation. We used the Fisher's exact test to determine whether administration of antibiotics before hospitalization that could affect relation and results between the causative strain and the gap from the first operation to second one. Also we used Fisher's exact test to evaluate prior hospitalization, previous MRSA infection, past medical history and weight bearing possibility. Moreover, multivariate logistic regression was applied to identify the significant predictors of MRSA infection by considering candidate variables with P-values of <0.05 in univariate analysis. We used backward stepwise selection procedure for multivariable model, and conducted likelihood ratio test to determine significance. We gained an ideal prediction cut-off value that can discriminate the two infection group by using receiver operating characteristic (ROC) curve for each predictor, and set cut-off values that satisfied over 80% of both sensitivity and specificity21). A P-value of <0.05 was considered significant. Statistical analysis was performed with use of PASW Statistic program (version 18.0; IBM Co., Armonk, NY, USA).
Demographic data, underlying disease, and the duration of symptom didn't show significant differences between two groups (Table 2). MRSA group have higher previous MRSA infection history than MSSA group (P=0.047). Prior empirical antibiotics usage which can affect value of laboratory test is not significantly difference (P=0.353) between two groups. MRSA group showed significant high neutrophil percentage (%) (MRSA: mean 76.4±9.4, MSSA: mean 63.4±10.7; P=0.002), ESR (MRSA: mean 79.6±29.2, MSSA: mean 40.2±19.1; P<0.001), and CRP (MRSA: mean 9.9±7.8, MSSA: mean 2.9±2.2; P<0.001). MRSA group also revealed longer duration for normalization of CRP (MRSA: mean 36.7±25.1 days, MSSA: mean 24.7±13.6 days; P=0.008), and longer duration for the second stage revision operation (MRSA: mean 60.9±32.1 days, MSSA: mean 47.6±6.2 days; P=0.012) than MSSA group. Vital sign (body temperature, heart rate), WBC count and duration for normalization of ESR (MRSA: mean 44.7±32.6 days, MSSA: mean 39.7±41.8 days; P=0.339) didn't show significant differences (Table 2).
For the duration of performing the second stage revision operation between MRSA group and MSSA group, it took 8.7 weeks in MRSA group, and 6.8 weeks in MSSA group on average. This difference was statistically significant (P=0.002) based on 7 weeks period (Table 3).
At MRSA group, 5 cases (13.9%) were recurred during follow up period. We conducted two-stage re-revision operation again for 2 patients and other 2 cases were treated by only intravenous vancomycin due to poor general condition. These 2 patients expired by sepsis, other one case was conducted limb amputation because of uncontrolled infection despite remove infected total knee replacement device. Three of 5 cases confirmed MRSA reinfection at culture test. One case was due to MRSA and methicillin resistance coagulase negative staphylococcus (MRCNS) coinfection and 1 case was by MRCNS pathogen. The mortality rate of MRSA group examined as 5.6% (2 of 36). In MSSA group, one case was found as reinfection 8 months after second stage revision. The pathogen confirmed as MRSA and conducted second stage revision again. There was no definite reinfection sign for 26 months follow up.
Multivariable regression analysis conducted targeting initial laboratory variables for the possibility of identifying significant predictors of MRSA infection. Significant differences between the two groups was found in neutrophil percentage (P=0.002), ESR (P<0.001), and CRP (P<0.001). We excluded duration for normalization of CRP and the duration for the second stage revision operation, because those were not proper variables for early infection predictors. As a result of applying ROC curve to the other three variables, they were located in confidence interval between 0.71 and 0.976, which means all those three variables have possibility of useful factors to discriminate MRSA and MSSA. We can set MRSA prediction cut-off value that satisfied over 80% of both sensitivity and specificity as a standard. As a result, when ESR was over 63.4 mm/hr and CRP was over 4.68 mg/dl, infections are more likely due to MRSA. For neutrophil percentage, we couldn't find out point that satisfied over 80% of both sensitivity and specificity.
We analyzed the differences between MSSA and MRSA infection based on laboratory test and vital signs that were conducted from patients who had infection after hip and knee arthroplasty. Neutrophil percentage, CRP, ESR, the duration for the second stage reimplantation operation, and the duration for normalization of CRP had statistically significant differences between the two groups. Authors thought that the differences came from higher toxicity of MRSA than that of MSSA, resulting in representation of higher inflammatory response7,8,9,10,11,12). In fact some studies have reported that MRSA infection showed higher ESR and CRP, and longer period until normalization of ESR and longer period of intravenous antibiotics treatment than MSSA infection. MRSA has higher proportion of Panton-Valentine leukocidin (PVL) gene which produces PVL material. PVL induces higher inflammatory response, resulting in high ESR and CRP11,22,23). This study targeted only patients who were proved staphylococcal infection. Actually, in case of patients who used antibiotics before diagnosis, the culture tests could come out negative. Also treatment of antibiotics before diagnosis and the period of antibiotic treatment could affect ESR and CRP results. However, in this study, whether patients have used antibiotics before didn't show significant difference between MRSA infection group and MSSA infection group (P=0.353). In recent study, some authors found the possibility of distinguishing MRSA and MSSA through initial ESR, CRP at initial stage9,10,24). But ESR, CRP have relative low specificity and also elevated by other site infection or inflammation25). So we cannot apply above data to distinguish MRSA between MSSA directly and need more study of clinical application.
Spangehl et al.26) reported that CRP usually normalized within 3 weeks after first stage operation. In our study, MSSA group which proper antibiotics used from the early stage normalized CRP at mean 24.7 days (3.5 weeks). On the other hand, MRSA group showed delaying normalization of CRP (mean 36.7 days, 5.2 weeks).
In this study, the length of time from the first mobile articulating spacer insertion to second revision operation of the MRSA infection group (60.9 days, 8.7 weeks on average) was significantly longer than MSSA infection group (47.0 days, 6.8 weeks) (P=0.012). This is because the culture test and antibiotic sensitivity test are conducted with clinical specimen gained from arthrocentesis or from the first mobile articulating spacer insertion operation, which normally takes a few days (4-7 days)10). We can interpret that the timing of administration of proper antibiotics gets delayed, which leads to longer treatment period to get rid of MRSA strains having stronger toxicity. Although there is no established standard for a proper time to perform second stage reimplantation operation, many studies reported that it is most desirable to perform second stage reimplantation operation in 6 weeks after the first operation on average27,28,29). Currently, some studies said that administer 4 to 8 weeks of intravenous antibiotics followed by a joint aspiration with the patient off of antibiotics for minimum of two weeks30). In this study, we performed second revision operation 6.8 weeks later on average in the MSSA infection group to which proper antibiotics from the early stage was applied. For MRSA infection group to which proper antibiotics from the early stage couldn't be applied, it took 8.7 weeks until second revision operation.
In current study, MRSA group have higher recurrence rate (13.9%) and mortality rate (5.6%) than MSSA group (4%, 0%) even with statistical insignificant. These findings are compatible with previous other researches that MRSA infection showed more complication and higher mortality rate. Based on above results, we can consider more active treatment such as early use of vancomycin for specific condition19,31,32,33). It would be able to reduce treatment period, complication rate, hospital cost.
Also we can consider repeated debridement or delayed second stage revision operation. Still lacking of evidence about the timing second stage revision, some authors suggest that much longer antibiotic treatment period is needed for resistant organism34). But there was another opinion that prolonged course of antibiotic therapy seems not to alter the incidence of recurrent or persistent infection35). Also there are no definite criteria for repeated debridement. We can evaluate the infection control status with frozen section at second stage operation. In current study, there was only one case which conducted redebridement procedure cause of still many PML cell under frozen section. But 5 cases of MRSA and one case of MSSA group recurred. So we need further study about the criteria of rebridement procedure and the infection control status such as interleukin-636), leukocyte esterase37). Furthermore we need lots of large study about PJI by resistant organism and design novel new treatment protocol for resistant organism PJI.
This study has a number of limitations in that it used retrospective study design, and it has a demerit that there are not enough cases for each infection strain, so it needs more supplementation. Also we were not able to determine genotypes of individual MSSA and MRSA isolates, which associate with toxicity. We evaluated only medical history which can affect inflammatory status (e.g., diabetes mellitus, hypertension, liver cirrhosis, and chronic renal failure), but it is not enough to consider all of host immunity factors. We only considered two-stage revision method due to chronic infection stage. So we cannot asses other treatment methods. We avoided information bias associated with analysis of incomplete data by excluding patients who had incomplete medical records. Furthermore, we avoided selection bias inclusion of patients with inconsistent diagnosis by excluding patients who did not have an exact, cultureproven diagnosis of Staphylococcus aureus infection.
PJI by MRSA showed frequent previous MRSA infection history, and higher neutrophil percentage, ESR and CRP at initial diagnosis. Also MRSA infection group have longer duration for the normalization of infection marker, longer treatment period. Furthermore the recurrence rate of MRSA infection is higher than those of MSSA infection. Aggressive treatment including early use of vancomycin, redebridement procedure should be considered for MRSA infection following arthroplasty.
Figures and Tables
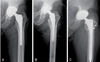 | Fig. 1X-ray images of a 62-year-old male who was diagnosed as periprosthetic joint infection of left total hip arthroplasty. (A) Hip anteroposterior X-ray at diagnosis. (B) We removed implants and inserted mobile articulating spacer. Methicillin-sensitive Staphylococcus aureus infection was proved through joint fluid and intraoperative tissue culture. (C) Second stage reimplantation was done at 6 weeks after mobile articulating spacer insertion. |
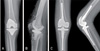 | Fig. 2X-ray images of a 75-year-old female patient who was diagnosed as periprosthetic joint infection of right total knee arthroplasty. (A) Knee anteroposterior X-ray at diagnosis. (B) We removed all of implants and inserted mobile articulating spacer. Methicillin-resistant Staphylococcus aureus infection was proved through joint fluid culture. (C) Second stage reimplantation was done at 10.3 weeks after mobile articulating spacer insertion. |
Table 2
Univariate Comparison between MRSA and MSSA Infection Groups

Values are presented as mean±standard deviation, number (%), or percent only.
*Diabetes mellitus, hypertension, liver cirrhosis, chronic renal failure.
MRSA: methicillin-resistant Staphylococcus aureus, MSSA: methicillin-sensitive Staphylococcus aureus, WBC: white blood cell, ANC: absolute neutrophil count, ESR: erythrocyte sedimentation rate, CRP: C-reactive protein.
References
1. Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004; 86:688–691.

2. Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008; 23:984–991.

3. Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001; 392:15–23.
4. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001; 392:315–318.

5. Bjerke-Kroll BT, Christ AB, McLawhorn AS, Sculco PK, Jules-Elysée KM, Sculco TP. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty. 2014; 29:877–882.

6. Ridgeway S, Wilson J, Charlet A, Kafatos G, Pearson A, Coello R. Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br. 2005; 87:844–850.

7. Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a metaanalysis. Clin Infect Dis. 2003; 36:53–59.

8. Goyal N, Miller A, Tripathi M, Parvizi J. Methicillin-resistant Staphylococcus aureus (MRSA): colonisation and pre-operative screening. Bone Joint J. 2013; 95-B:4–9.
9. Hawkshead JJ 3rd, Patel NB, Steele RW, Heinrich SD. Comparative severity of pediatric osteomyelitis attributable to methicillin-resistant versus methicillin-sensitive Staphylococcus aureus. J Pediatr Orthop. 2009; 29:85–90.

10. Ju KL, Zurakowski D, Kocher MS. Differentiating between methicillin-resistant and methicillin-sensitive Staphylococcus aureus osteomyelitis in children: an evidence-based clinical prediction algorithm. J Bone Joint Surg Am. 2011; 93:1693–1701.

11. Saavedra-Lozano J, Mejías A, Ahmad N, et al. Changing trends in acute osteomyelitis in children: impact of methicillin-resistant Staphylococcus aureus infections. J Pediatr Orthop. 2008; 28:569–575.
12. Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res. 2007; 461:48–53.

13. Parvizi J, Pawasarat IM, Azzam KA, Joshi A, Hansen EN, Bozic KJ. Periprosthetic joint infection: the economic impact of methicillin-resistant infections. J Arthroplasty. 2010; 25:6 Suppl. 103–107.
14. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996; 78:512–523.

15. Workgroup Convened by the Musculoskeletal Infection Society. New definition for periprosthetic joint infection. J Arthroplasty. 2011; 26:1136–1138.
16. Estes CS, Beauchamp CP, Clarke HD, Spangehl MJ. A two-stage retention d?bridement protocol for acute periprosthetic joint infections. Clin Orthop Relat Res. 2010; 468:2029–2038.

17. Van Kleunen JP, Knox D, Garino JP, Lee GC. Irrigation and débridement and prosthesis retention for treating acute periprosthetic infections. Clin Orthop Relat Res. 2010; 468:2024–2028.

18. Koo KH, Yang JW, Cho SH, et al. Impregnation of vancomycin, gentamicin, and cefotaxime in a cement spacer for two-stage cementless reconstruction in infected total hip arthroplasty. J Arthroplasty. 2001; 16:882–892.

19. Meehan J, Jamali AA, Nguyen H. Prophylactic antibiotics in hip and knee arthroplasty. J Bone Joint Surg Am. 2009; 91:2480–2490.

20. Della Valle CJ, Bogner E, Desai P, et al. Analysis of frozen sections of intraoperative specimens obtained at the time of reoperation after hip or knee resection arthroplasty for the treatment of infection. J Bone Joint Surg Am. 1999; 81:684–689.

21. Pepe MS. The statistical evaluation of medical tests for classification and prediction. 1st ed. Oxford, USA: Oxford University Press;2004.
22. Bocchini CE, Hulten KG, Mason EO Jr, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006; 117:433–440.

23. Lo WT, Tang CS, Chen SJ, Huang CF, Tseng MH, Wang CC. Panton-Valentine leukocidin is associated with exacerbated skin manifestations and inflammatory response in children with community-associated staphylococcal scarlet fever. Clin Infect Dis. 2009; 49:e69–e75.

24. Jung WJ, Kang YA, Park MS, et al. Prediction of methicillin-resistant Staphylococcus aureus in patients with non-nosocomial pneumonia. BMC Infect Dis. 2013; 13:370.
25. Berbari E, Mabry T, Tsaras G, et al. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am. 2010; 92:2102–2109.

26. Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999; 81:672–683.

27. Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983; 65:1087–1098.

28. Petty W, Bryan RS, Coventry MB, Peterson LF. Infection after total knee arthroplasty. Orthop Clin North Am. 1975; 6:1005–1014.

29. Whittaker JP, Warren RE, Jones RS, Gregson PA. Is prolonged systemic antibiotic treatment essential in two-stage revision hip replacement for chronic Gram-positive infection? J Bone Joint Surg Br. 2009; 91:44–51.

30. Aggarwal VK, Rasouli MR, Parvizi J. Periprosthetic joint infection: Current concept. Indian J Orthop. 2013; 47:10–17.

31. Dubée V, Zeller V, Lhotellier L, et al. Continuous high-dose vancomycin combination therapy for methicillin-resistant staphylococcal prosthetic hip infection: a prospective cohort study. Clin Microbiol Infect. 2013; 19:E98–E105.

32. Liu C, Kakis A, Nichols A, Ries MD, Vail TP, Bozic KJ. Targeted use of vancomycin as perioperative prophylaxis reduces periprosthetic joint infection in revision TKA. Clin Orthop Relat Res. 2014; 472:227–231.

33. Nickinson RS, Board TN, Gambhir AK, Porter ML, Kay PR. The microbiology of the infected knee arthroplasty. Int Orthop. 2010; 34:505–510.

34. Kuzyk PR, Dhotar HS, Sternheim A, Gross AE, Safir O, Backstein D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection: techniques, controversies, and outcomes. J Am Acad Orthop Surg. 2014; 22:153–164.

35. Hoad-Reddick DA, Evans CR, Norman P, Stockley I. Is there a role for extended antibiotic therapy in a two-stage revision of the infected knee arthroplasty? J Bone Joint Surg Br. 2005; 87:171–174.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download