Abstract
Purpose
Patients who have secondary hip osteoarthritis as sequelae of Legg-Calvé-Perthes disease (LCPD) are severe deformities of femoral head and acetabulum. A few studies have presented that the clinical results and risks associated with total hip arthroplasty (THA) for patients with a history of LCPD were not satisfactory. In this study, we reported the radiographic and clinical outcomes of THA in patients with sequelae of LCPD.
Materials and Methods
Between March 2007 and May 2012, 23 hips (23 patients) underwent cementless THA and were followed up at least 2 years after surgery. There were 11 male patients and 12 female patients with an average age of 49.2 years old (range, 25 to 69 years old), and the average follow up period was 40.8 months (range, 24 to 84 months). The clinical and radiological evaluations were performed.
Results
The Harris hip score improved from 48.3 points preoperatively to 92.4 points at the time of the last follow-up. The shortening of affected limb was improved from -1.6 cm to 0.2 cm. The complications included one case of sciatic nerve palsy that developed after extensive lengthening of lower extremity, three cases of intraoperative femur fractures. There was no component loosening.
Conclusion
Fractures and motor nerve palsies may be more frequent in this population. Careful preoperative planning should be performed to overcome the technical pitfalls. If overcoming this early complication, the clinical and radiological evaluations showed excellent outcomes at average 40-month follow-ups.
Legg-Calvé-Perthes disease (LCPD) is represented by osteonecrosis of the femoral head during childhood, which typically develops in children aged 3 to 10 years1,2,3,4). The onset age of the patient, development of osteonecrosis and the pathogenesis of femoral head necrosis are the most important factors determining the success of initial treatment received during childhood. Despite receiving treatment, some patients develop early-onset secondary osteoarthritis requiring total hip arthroplasty (THA). A recent prospective series found a 5% rate of THA at 20 years after nonoperative treatment of LCPD5). The outcomes of THA for patients with osteonecrosis of the femoral head are less satisfactory than those for patients with primary osteoarthritis6,7,8,9).
The technical difficulties of THA in patients who have LCPD are unique to the condition where the femoral head develops multi-planar deformities10). The acetabulum is typically shallow, enlarged, and retroverted11), and the head of the femur flattens and widens, becoming incongruent with the acetabulum. The greater trochanter becomes elongated, and the neck of the femoral head is short due to growth disturbance. Secondary osteoarthritis of the hip occurs early in most patients, especially in those classified as Stulberg types III-V3). THA is often complicated by previous surgical procedures undertaken during childhood. Previous surgical procedures (particularly femoral osteotomies) make THA more difficult. As the normal proximal femoral anatomy is altered, femoral component implantation becomes more challenging12).
To our knowledge, a few articles have reported the outcomes of THA in patients with the sequelae of LCPD. The aim of this study was to review the results of THA in patients with LCPD with at least follow-up of two years.
We retrospectively reviewed 23 patients (23 hips) who underwent primary THA performed between March 2007 and May 2012. All patients had secondary osteoarthritis due to the sequelae of LCPD. All data for this study were retrieved from our institution's database; we did not see or contact patients specifically for this study (KC14RISI0749).
There were 11 men and 12 women with a mean age of 49.2 years (range, 25-69 years) at the time of the arthroplasty. The mean body weight was 67.1 kg (range, 49-91 kg), and the mean body mass index was 27.3 (range, 22.1-37.5). The minimum follow-up was 2 year (mean, 40.8 months; range, 24-84 months). All surgeries were performed through a modified posterolateral approach with preserving pyriformis tendon13). All prostheses were used BENCOX® hip system (Corentec, Cheonan, Korea) except one (Stryker, Kalamazoo, MI, USA), and all had a neck taper of 12/14 mm and a 32 or 36 mm ceramic head (Table 1).
Clinical results were evaluated on the 3, 6 and 12 months postoperatively, and every following postoperative year. Hips with a score of ≥90 points were defined as excellent, 80-89 as good, 70-79 as fair, and <70 as poor14). The presence of postoperative thigh pain was also examined. The relationship of thigh pain with radiolucent lines observed on radiographs, cortical hypertrophy, and pedestal formation was investigated. For radiological assessment, subsidence of femoral components, stress shielding, cortical hypertrophy, periprosthetic reactive lines, and osteolysis were examined by dividing the proximal femur into Gruen zones15) on anteroposterior and lateral radiographs around the femoral components. Femoral component fixation was graded as bony stable, fibrous stable, or unstable according to the criteria described by Engh et al.16). For the femoral component, subsidence of >5 mm was classified as loosening according to the method of Callaghan et al.17). Cortical hypertrophy was defined as an increase in the diameter of the cortex measured at the point of maximum hypertrophy. A reactive line was defined as a parallel radiolucent line adjacent to the prosthesis. Movement of the acetabular cup was compared using radiographs taken in the immediate postoperative period and at the final follow-up based on the anterior and posterior views of the hip. According to the method of Dorr et al.18), the distances between the acetabular component and Kohler's line and the teardrop were measured. Osteolysis around the acetabular cup was presented as DeLee and Charnley zones19). The acetabular cup was defined as loosening if there was movement in the position of the cup of more than 2 mm vertically, medially, or laterally, if the radiolucent lines were widened more than 2 mm in the anterior, posterior, or lateral radiographs of the acetabular cup, or if the inclination angle changed by more than 5 degrees.
We assessed the pre-operative and post-operative leg length discrepancy (LLD) 6 months postoperatively using the method described by Ranawat et al.20) On an anteroposterior radiograph of the pelvis, we drew a horizontal reference line through the inferior aspect of the teardrops (the perpendicular distance between the reference line and lesser trochanter).
We identified the presence of complications including sciatic nerve palsy, infection, dislocation, fractures in the ceramic head and liner, heterotopic ossification, and squeaking. We performed statistical analyses using SPSS® (version 11.5; SPSS Inc., Chicago, IL, USA).
The average Harris hip score was improved from 48.3 points (range, 42-67) preoperatively to 92.4 points (range, 79-97) postoperatively. There were 15 excellent cases (65.2%), 7 good cases (30.4%), 1 fair case (4.3%), and no poor cases. No patients complained of thigh pain. In the final follow-up, all hips showed stable fixation. Of these, no hip had no osteolysis, loosening subsidence around the implants (Fig. 1). The average LLD was improved -1.5 cm (range, -2.5--0.7 cm) preoperatively to 0.3 cm (range, -0.1-0.7 cm) postoperatively, and the average limb lengthening after operation was 1.8 cm (range, 0.9-2.5 cm). The mean operation time was 94 min (range, 55-130 min), and total amount of drainage was 443.3 mL (range, 265-720 mL).
Postoperative sciatic nerve palsy in one hip (4.3%) with postoperative sciatic nerve palsy was occurred due to over-lengthening, so we revised the THA with medicalization of acetabular cup and exchange with smaller sized stem (Fig. 2). After revisional surgery, sciatic nerve palsy was improved to Grade IV at the last follow-up. There was no dislocation, infection, and heterotopic ossification.
Intra-operative fracture in three hips (13.0%) was occurred during stem insertion, so these fractures were fixed with wires and cables, and weight bearing was not permitted until 6 weeks. There was no stem subsidence in fracture cases.
This study evaluates a population of 23 patients treated in childhood for LCPD who later underwent THA. The goal of childhood LCPD treatment is to prevent end-stage arthritis, which today typically is addressed with THA. The present report focuses on a group of patients with LCPD who went on to THA as a result of their pediatric hip diagnosis. We found that although survivorship of these hip reconstructions was generally good, serious complications were frequent.
In our series, modern cementless implants had acceptable survivorship of 95.7% at 40-month followup. The only previous report of THA survivorship in patients with a history of LCPD cites a 96% survivorship at 15 years for primarily ingrowth components21). Takenaga et al.22) report 86% 10- to 15-year survivorship for cementless implants in patients younger than age 50 years. Thus, our series compares favorably with other reports of THA in a younger cohort of patients.
However, we found a high rate of neurologic injury in patients with a history of LCPD undergoing THA. This is much higher than the reported risk of 0.17% of patients undergoing arthroplasty for any cause23). Although hip dysplasia has long been known to be a risk factor for neurologic complications, LCPD has not typically been thought of as a risk factor for sciatic nerve palsy. One small case series also reports a high rate of neurologic deficit in patients with LCPD undergoing arthroplasty (6%)22). In our case, LLD was pre-operatively measured about 1.3 cm (Fig. 2A), and the affected limb was post-operatively over-lengthened about 7 mm (Fig. 2B). The patients complained severe neurogenic pain on the affected leg, and motor weakness (Grade 0). Perhaps the longstanding nature of the shortened limb may put the patient at increased risk of neurologic injury compared with the typical patient with osteoarthritis. The surgeon should consider to over-lengthen the limb for patients with LCPD with severe length leg discrepancy.
A high risk of intra-operative femoral fracture (13% in our series) was observed compared with 3% of patients undergoing arthroplasty for any cause23). A characteristic deformity of femur in these patients would make the fracture during stem insertion. A press-fit femoral component could be difficult to achieve with modular implants where there is severe dysplasia in the proximal femur. The custom-made femoral design optimizes metaphyseal bone contact in the proximal femur, an important requirement in cementless THAs24). The bespoke stem design also allows correction of marked femoral neck anteversion, a common feature in patients with LCPD25), and accommodation of the proximal femoral deformities created by previous surgery, ensuring a metaphyseal press-fit. Careful preoperative evaluation, including the use of computed-tomography based systems26), can reduce the overall intraoperative risks.
There are several limitations to the study. The fist, data regarding the childhood treatment course and early radiographs are limited. Thus, we cannot attempt to correlate pediatric treatment of the disease or disease severity with our reported results of THA, because these data were deficient. The second, this study is a retrospective one performed in a small cohort observed patient with short-term follow-up. The long term follow up must be necessary. The third, it does not have control group of primary THA due to primary osteoarthritis.
Fractures and motor nerve palsies may be more frequent in this population. Careful preoperative planning should be performed to overcome the technical pitfalls. If overcoming this early complication, the clinical and radiological evaluations showed excellent outcomes at average 40-month follow-ups.
Figures and Tables
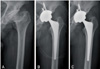 | Fig. 1(A) This anteroposterior radiography shows secondary osteoarthritis caused by sequelae of Legg-Calvé-Perthes disease in a 57-year-old male. (B) The immediate postoperative radiography shows good positioning of the prosthesis. (C) After 5 years, the radiograph shows no osteolysis around the right acetabular and femoral component, and stable components with proper bony ingrowth. |
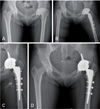 | Fig. 2(A) This anteroposterior radiography shows secondary osteoarthritis caused by sequelae of Legg-Calvé-Perthes disease in a 35-year-old female. Preoperatively, leg length discrepancy was measured about 1.3 cm. (B) The immediate postoperative radiography shows that the affected limb was over-lengthened about 7 mm. The patients complained severe neurogenic pain on the affected leg, and motor weakness (Grade 0). (C) The total hip arthroplasty was revised with medicalization of acetabular cup and exchanging with smaller sized stem. Postoperatively, leg length discrepancy was not examined. (D) After 2 years, the radiograph shows no osteolysis around the right acetabular and femoral component, and the sciatic nerve palsy was improved (Grade IV). |
References
1. Gower WE, Johnston RC. Legg-Perthes disease. Long-term follow-up of thirty-six patients. J Bone Joint Surg Am. 1971; 5:759–768.
2. Kelly FB Jr, Canale ST, Jones RR. Legg-Calvé-Perthes disease. Long-term evaluation of non-containment treatment. J Bone Joint Surg Am. 1980; 62:400–407.

3. Stulberg SD, Cooperman DR, Wallensten R. The natural history of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 1981; 63:1095–1108.

5. Larson AN, Sucato DJ, Herring JA, et al. A prospective multicenter study of Legg-Calvé-Perthes disease: functional and radiographic outcomes of nonoperative treatment at a mean follow-up of twenty years. J Bone Joint Surg Am. 2012; 94:584–592.
6. Al-Khateeb H, Kwok IH, Hanna SA, Sewell MD, Hashemi-Nejad A. Custom cementless THA in patients with Legg-Calvé-Perthes Disease. J Arthroplasty. 2014; 29:792–796.
7. Baghdadi YM, Larson AN, Stans AA, Mabry TM. Total hip arthroplasty for the sequelae of Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 2013; 471:2980–2986.

8. Radl R, Hungerford M, Materna W, Rehak P, Windhager R. Higher failure rate and stem migration of an uncemented femoral component in patients with femoral head osteonecrosis than in patients with osteoarthrosis. Acta Orthop. 2005; 76:49–55.

9. Saito S, Saito M, Nishina T, Ohzono K, Ono K. Long-term results of total hip arthroplasty for osteonecrosis of the femoral head. A comparison with osteoarthritis. Clin Orthop Relat Res. 1989; 244:198–207.
10. Gent E, Clarke NM. Joint replacement for sequelae of childhood hip disorders. J Pediatr Orthop. 2004; 24:235–240.

11. Sankar WN, Flynn JM. The development of acetabular retroversion in children with Legg-Calvé-Perthes disease. J Pediatr Orthop. 2008; 28:440–443.

12. Kawasaki M, Hasegawa Y, Sakano S, Masui T, Ishiguro N. Total hip arthroplasty after failed transtrochanteric rotational osteotomy for avascular necrosis of the femoral head. J Arthroplasty. 2005; 20:574–579.

13. Kim YS, Kwon SY, Sun DH, Han SK, Maloney WJ. Modified posterior approach to total hip arthroplasty to enhance joint stability. Clin Orthop Relat Res. 2008; 46:294–299.

14. Harris WH. Preliminary report of results of Harris total hip replacement. Clin Orthop Relat Res. 1973; 95:168–173.

15. Gruen TA, McNeice GM, Amstutz HC. "Modes of failure" of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res. 1979; 141:17–27.
16. Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br. 1987; 69:45–55.

17. Callaghan JJ, Salvati EA, Pellicci PM, Wilson PD Jr, Ranawat CS. Results of revision for mechanical failure after cemented total hip replacement, 1979 to 1982. A two to fiveyear follow-up. J Bone Joint Surg Am. 1985; 67:1074–1085.

18. Dorr LD, Wan Z, Song M, Ranawat A. Bilateral total hip arthroplasty comparing hydroxyapatite coating to porous-coated fixation. J Arthroplasty. 1998; 13:729–736.

19. DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976; 121:20–32.

20. Ranawat CS, Rao RR, Rodriguez JA, Bhende HS. Correction of limb-length inequality during total hip arthroplasty. J Arthroplasty. 2001; 16:715–720.

21. Traina F, De Fine M, Sudanese A, Calderoni PP, Tassinari E, Toni A. Long-term results of total hip replacement in patients with Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 2011; 93:e25.

22. Takenaga RK, Callaghan JJ, Bedard NA, Liu SS, Klaassen AL, Pedersen DR. Cementless total hip arthroplasty in patients fifty years of age or younger: a minimum ten-year follow-up. J Bone Joint Surg Am. 2012; 94:2153–2159.

23. Farrell CM, Springer BD, Haidukewych GJ, Morrey BF. Motor nerve palsy following primary total hip arthroplasty. J Bone Joint Surg Am. 2005; 87:2619–2625.

24. Bargar WL. Shape the implant to the patient. A rationale for the use of custom-fit cementless total hip implants. Clin Orthop Relat Res. 1989; 249:73–78.
25. Upadhyay SS, Burwell RG, Moulton A. Femoral anteversion in Perthes' disease with observations on irritable hips. Application of a new method using ultrasound. Clin Orthop Relat Res. 1986; 209:70–76.
26. Toni A, Traina F, Viceconti M. Computer-assisted tridimensional preoperative planning in hip revision surgery. Chir Organi Mov. 2003; 88:273–280.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download