Rapidly destructive coxarthrosis (RDC) is a rare syndrome that involves severe pain in the hip and claudication with the etiology remaining elusive. Initial radiographic findings show either normal anatomy or mild arthritis, but rapid destruction of the femoral head occurs within months of the onset of clinical manifestations, which include severe pain and rapid bone destruction. Thus, this condition has to be distinguished from septic arthritis, rheumatoid arthritis, bone tumor and other pathologies1). In previous case reports, rapid destruction of the hip joint along with an enlarged iliopsoas bursa was seen in patients with rheumatoid arthritis receiving treatment2). No rapid destruction of the hip joint associated with an enlarged iliopsoas bursa has been reported in healthy individuals to date. We encountered a rare case of rapid destruction of the femoral head along with an enlarged iliopsoas bursa which was misdiagnosed as an infection in a healthy man. This condition evolved into progressive hip joint destruction within 3 months. The report of this case with a brief review of literature follows.
CASE REPORT
A 72-year-old man (weight 55 kg, height 168 cm, body mass index 19.5) was referred to our department for interdisciplinary treatment with the chief complaint of painful limited range-of-motion of the left hip joint and a mass in the left anterior iliac bone. He was otherwise healthy and had no specific medical history. Initially, he underwent symtomatic treatment with medications and nerve block after being diagnosed to have lumbar spinal stenosis with back and hip pain manifested for 3 months. However, the hip condition deteriorated to a degree that made him unable to walk a month prior to his last referral. In physical examination, a 10×18 cm mass was palpated around the inguinal area and iliac crest on the left anterior iliac bone. The mass was not tender nor local heatness. Hip joint pain increased in the last two weeks prior to his presentation along with a rapid increase in the size of the mass. This made him unable to walk or stand.
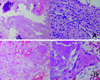
Plain lumbar radiographs taken at another department 3 months prior to his presentation had revealed non-specific findings in the left hip joint (Fig. 1A). In contrast, plain radiographs of the hip taken at the time of referral revealed aggressive joint destruction including femoral head collapse and subchondral sclerosis of the acetabulum (Fig. 1B). The patient had no risk factors for avascular necrosis of the femoral head, which include excessive alcohol drinking and steroid use. He had intermittent mild fever at about 37℃ at night. In laboratory tests, white blood cell count was 11.7×103/mm3 (normal range, 4.0-10.0×103/mm3), erythrocyte sedimentation rate was 48 mm/hr (normal range, 15 mm/hr), C-reactive protein (CRP) was 8.43 mg/L (normal range, <5 mg/L), and rheumatoid arthritis (RA) factor was 10.1 IU/mL (normal range, <14 IU/mL). Magnetic resonance imaging (MRI) revealed an enlarged iliopsoas bursa connected to the left hip cyst and hip joint effusion (Fig. 1C, D). In ultrasound-guided aspiration biopsy, 40 mL of an opaque serous fluid was aspirated, but the size of the mass was not reduced noticeably. No bacteria were detected in the culture of the aspirated fluid. The patient underwent surgery in a lateral decubitus position under general anesthesia. To confirm the decompression and composition of the enlarged iliopsoas bursa, an incision was made on the anterior aspect of the iliac crest of the mass and the fluid of bursa was drained. Sufficient curettage of bursal cysts was performed. Hip joint was accessed via anterolateral approaches by maintaining adequate space with an incision on the iliac crest. On surgical inspection, iliopsoas bursa extended close to the joint capsule anteriorly. Internal joint was approached after drainage along with sufficient curettage of the bursa. Surgical approach to the hip joint was initially made to perform hip arthroplasty if an infection was not detected. Frozen section biopsy was taken to identify the possible association of bone destruction with infectious diseases and to clarify the cause of the RDC. Inside the hip joint, destruction of the femoral head and excessive synovial fluid were detected. The articular cartilage of the acetabulum was damaged. Bacterial cultures were requested for anterior iliac crest, peripheral joint capsule and joint fluid. Frozen section biopsy was taken from the soft tissues in the bursa and joint (Fig. 2A). It showed acute inflammatory cells, ectopic calcification and granulomatous inflammation. However, there was no clear finding of an infectious disease such as bacterial clusters or tuberculosis. Initial hip arthroplasty should have been performed with caution due to the presence of an opaque serous fluid and patient's preoperative conditions of intermittent mild fever and CRP increase. After the insertion of a prosthesis and antibiotic-loaded acrylic cement (PROSTALAC), the results of bacterial culture and biopsy were confirmed. The initial operation was completed to perform a secondary operation (Fig. 3). Two weeks after the operation, there were no findings of an infection in the studies requested at the time of the initial surgery. Femoral head biopsy in the first operation showed necrosis of the osseous tissue along with presence of osteoclasts and osteoblasts regulating endosteal bone formation around osteonecrotic tissues. Based on these findings, avascular necrosis became the presumed diagnosis for the rapid destruction of the femoral head (Fig. 2B). There was no fever or surgical wound infection in physical examination and CRP was normal, therefore, cementless total hip arthroplasty was conducted as the secondary surgery through the existing incision (Fig. 4A). In the second operation, recurrence of bursa or mucous retention was not observed. The PROSTALAC was removed easily, and there were no findings suspicious of an infection in the acetabulum or the femoral marrow cavity. The mass did not recur and the patient maintained a stable artificial hip joint and normal ambulation at 12 months postoperatively and at the final follow-up (Fig. 4B).
DISCUSSION
RDC is manifested with severe pain in the hip and claudication. It results in a rapid destruction of the femoral head within months from the onset of clinical manifestations. The etiology is still unclear. This rare condition is known to be associated with osteoarthritis3) or rheumatoid arthritis, but has to be discriminated from septic arthritis or rheumatoid arthritis. Our patient had no history of nonsteroidal anti-inflammatory drug4) or steroid use, and was healthy enough to carry out farm work.
Along with symptoms and findings in the physical examination being compatible with RDC, simple radiographs revealed destruction of the femoral head, the acetabular surface and loss of joint space. Extensive edema-like pattern in the bone marrow of the femoral head and neck, flattening of the femoral head and cystic-like subchondral defects are known as significant characteristics of RDC in MRI5). Based on plain radiographs and MRI findings, RDC can be distinguished from septic arthritis, rheumatoid arthritis and avascular necrosis of the femoral head. Unlike septic arthritis, RDC is characterized by indistinct junction of bone destruction in simple radiography. In MRI, secondary spur formation and inflammatory lesions limited to joints are seen1). In our patient, infectious arthritis was not detected in bone marrow and negative results were found in the aspiration biopsy and microbiological smear and culture of the aspirated fluid. Although the clinical manifestations of rheumatoid arthritis in simple radiography are comparable with RDC, pain is not seen in RA. Patients with RA frequently have a history of syphilis, diabetes mellitus, syringomyelia, spinal cord injury, and the onset site is less frequently the hip joint. Lee et al.6) suggested that necrosis of femoral head or femoral edema, thickened synovial lining with distinctive contrast enhancement, osteochondral loose bodies and bone marrow edema in the acetabular region are detected in the MRI images of RDC. These are distinctive manifestations discriminating RDC from avascular necrosis of the femoral head in Ficat stage IV. We confirmed destruction of the femoral head and acetabular surface and loss of joint space in simple radiographs. Therefore, we diagnosed the patient to have RDC based on the findings of extensive edema-like pattern in the bone marrow, flattening of the femoral head and cystic-like subchondral defects in MRI.
Since our patient had also an enlarged iliopsoas bursa, it was difficult to distinguish RDC from infectious diseases. To make this distinction, the composition of aspirated fluid was examined and no microorganism was detected in bacterial culture. However, we could not completely exclude the risk of an infectious disease without performing hip arthroplasty because of the small elevation in CRP, aspects of aspirated fluid and surgical field. Therefore, the final operation was conducted after confirming the results of biopsies from different tissues and mucus culture.
The iliopsoas bursa is a structure with an average size of 3-6 cm and is situated anterior to the hip joint capsule and beneath the iliopsoas muscle in 98% of normal individuals. Since the anterior joint capsule of the iliopsoas bursa is relatively thin and weak, connection between the capsule and the bursa is known to exist to in nearly 15% of the normal hip joints and 40% of osteoarthritic hip joints. In the presence of this connection, production of excessive synovial fluid in hip joint diseases may increase the size of the iliopsoas bursa7). When the iliopsoas bursa is enlarged anteriorly, it grows between iliopsoas and pectineus muscles and develops as a mass in the inguinal region from the lateral side of femoral artery. Enlargement of the iliopsoas bursa needs to be differentiated from inguinal hernia, lymphadenopathy, lymphoma, vascular abnormality, iliopsoas abscess and aneurysm8). As our patients shows, enlargement of the iliopsoas bursa could also be seen due to other reasons. Overuse of young athletes, heavy labor, trauma and repetitive friction are other possible causes for enlargement of the iliopsoas bursa9). Iliopsoas bursa could also be enlarged due to direct connection between the hip joint and bursa as anterior part of the joint capsule becomes weak in presence of arthritis in the hip joint, increased pressure inside the joints due to increased synovial fluid, or production of excessive synovial fluid from synovial membranes2).
In our patient, RDC occurred over a short period of time and involved an enlarged iliopsoas bursa. Palpation was done 2 weeks before the patient referral for interdisciplinary treatment; however, the actual onset of his condition is unclear. Furthermore, iliopsoas bursitis is a calcified lesion that could be detected in the lesser trochanteric region in simple radiographs and computed tomography images of anterior-posterior views of the hip joint in patients with enlarged iliopsoas bursa. It is anticipated to be associated with chondrocalcinosis, which is caused by deposition of calcium pyrophosphate dehydrate (CPPD) crystals and is shown to incur RDC10). Consequently, it was difficult in our patient to determine whether RDC had occurred due to the enlargement of the iliopsoas bursa or iliopsoas bursitis. Since this is a single patient report, we were unable to verify whether RDC was caused by iliopsoas bursitis or not. When rapid destruction of the hip joint is associated with an iliopsoas bursa, it is difficult to initially perform hip arthroplasty because of the risk of an unidentified infection, which may lead to re-operations like our presented case. Therefore, it could be acceptable to initially perform hip arthroplasty despite the presence of rapid destruction of the hip joint associated with an iliopsoas bursa, if there are no signs of infections in a series of preoperative tests including bacterial culture.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download