Abstract
Purpose
Methods
Results
Conclusion
Figures and Tables
Table 1
Risk of Bias for RCT & NRCT
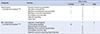
| Categories | Domain | Risk of bias | ||
|---|---|---|---|---|
| Unclear | Low | High | ||
| Randomized controlled trial studies18,19,20) | Random sequence generation | 1 | 1 | 1 |
| Allocation concealment | - | 3 | - | |
| Blinding of participants and personnel | 1 | 2 | - | |
| Blinding of outcome assessment | 1 | 2 | - | |
| Incomplete outcome data | - | 3 | - | |
| Selective reporting | - | 3 | - | |
| Other bias | - | 3 | - | |
| Non randomized controlled trial studies21,22,23,24,25,26) | Selection of participants | - | 1 | 5 |
| Confounding variables | 6 | - | - | |
| Measurement of exposure | - | 6 | - | |
| Blinding of the outcome assessments | - | 6 | - | |
| Incomplete outcome data | - | 6 | - | |
| Selective outcome reporting (reporting bias) | - | 6 | - | |
Table 2
General Characteristics of Studies

No= Reference number.; BC= Breast cancer; LC= Lung cancer; CRC= Colorectal cancer; OPD= Outpatient department; PA= Physical activity; QoL= Quality of life; BW= Body weight; UK= United kingdom; RCT= Randomized controlled trial; NRCt= Non-randomized controlled trial; Tx= Treatment ; Exp.= Experimental group; Cont.= Control group; App.= Application; F/U= Follow-up; VS= Versus; Qoc= Quality of care; ASyMS= Advanced symptom management system.
Table 3
General Characteristics of Application
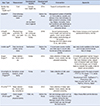
| App. Type | Measurement | Development country | Development Year | Intervention | Appendix |
|---|---|---|---|---|---|
| Mobile App (QoC Health Inc)17) | Cost | Canada QoC Health Inc. | 2014 | Support postoperative care | |
| ASyMS18) | Chemotherapy related symptoms | UK (six Scotland/one England) | Before the Study | Management system (ASyMS©) on the incidence, severity and distress of six chemotherapy-related symptoms (nausea, vomiting, fatigue, mucositis, handfoot syndrome and diarrhoea) in patients with lung, breast or colorectal cancer | |
| mHealth ‘smart after care19) | Physical measurement, PA, QOL, user satisfaction | Korea BIT Computer Co., Ltd., Seoul, Korea | 2016 |
Governmental projects, Home health care services for gastric, colon cancer patient who receive chemotherapy after surgery The App let know patient what to do, diet control. |
http://www.monews.co.kr/news/articleView.html?idxno=94764 |
| mobile app20) | Daily functional activity, symptoms | Switzerland | Before the Study | A novel open-source mobile and Web app to record daily functional activity and adverse events. | app was made available in the Apple and Google Android stores free of charge |
| Dugun Dugun21) | Sleep satisfaction, mood, anxiety | Korea | 2012 | Data collection of sleep satisfaction, mood, anxiety and depression screening | A mobile application named ‘Dugun-Dugun’ (Pit a pat) was Developed by the Asan Medical Center, Department of Mental Health and Breast Endocrine Surgery and U-health team to evaluate the quality of life for breast cancer patients. |
| Uncertainty Intervention Program23) | Symptom, social support, uncertainty | Korea | 2013 | Data collection of diet, exercise, pain |
Language used in the apps program : PHP 5.2.16, jQuery 1.7.2, javascript Development tools : NetBeans 7.1, Photoshop CS5 Web browser test :IE7, IE8, IE9, FireFox, Chrome, Safari |
| Pit a Pat22,24,25) | Sleep satisfaction, mood, anxiety, compliance | Korea | 2012 | Data collection of sleep satisfaction, mood, anxiety and depression screening | =DugunDugun |
| mHealth (Fit bit app)26) | Body weight, diet, physical activity | Fitbit, Inc. | Before the Study | Set and manage goals such as number of steps, distance, and calories during the day Manage weight while checking calorie intake per day through the food record | https://www.fitbit.com/kr/devices |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download