Abstract
Purpose
The purpose of the present study was to assess factors associated with quality of life (QOL) and to determine whether anxiety and depression are predictive of QOL in patients with advanced gastrointestinal cancer at initial diagnosis and during the treatment process.
Methods
One hundred and twenty patients with gastrointestinal cancer requiring palliative chemotherapy were enrolled.
Results
At baseline, depression, performance status, and anxiety accounted for 55.0% (p<.001) of the variance in global health status score, depression accounted for 22.0% (p<.001) of the variance in functional scales score, and anxiety accounted for 19.0% (p<.001) of the variance in symptom scales score. At 3 months, depression, pain, and performance status accounted for 72.0% (p<.001) of the variance in global health status score, 76.0% (p<.001) of the variance in functional scales score, and 74.0% (p<.001) of the variance in symptom scales score.
Conclusion
Anxiety and depression were significant predictive factors of QOL in patients with advanced gastrointestinal cancer. Depression and performance status were significant predictive factors of QOL at both baseline and 3 months, and anxiety and pain were significant predictive factors of QOL at baseline and 3 months, respectively.
The worldwide incidence of gastrointestinal cancer is relatively high compared with other cancers.1) Gastric cancers occur most frequently in males, and gastrointestinal cancers, including stomach, colon, rectum, liver, pancreas, and gallbladder cancer, are among the ten leading types of new cancer cases in both males and females in Korea.2) Although the overall incidence of advanced or metastatic gastrointestinal cancer has been lowered through early screening, and patients with early gastrointestinal cancer can be cured by surgical resection, a large majority of patients experience a relapse after surgical resection or are initially diagnosed with locally advanced unresectable or metastatic disease.23) For patients with advanced gastrointestinal cancer requiring palliative therapy, the 5-year survival rate has been reported to be 1.7~19.0%.234)
Traditionally, the primary goal of cancer therapy has been to decrease tumor size and increase overall survival rate. However, indicators of the efficacy of cancer therapy have expanded to include not only survival rate, but also quality of life (QOL), and QOL is an independent prognostic factor for survival rate in cancer patients.56) QOL is an important outcome measure in all cancer patients, and is particularly critical in patients with metastatic cancer as their treatment is limited to palliative therapy and cure is no longer the goal. QOL is a multidimensional concept that includes physical, psychological, social and cognitive functioning, and the impact of illness and treatment on the patient's life.78) Psychological distress such as anxiety or depression is common in cancer patients, reduces QOL, and increases mortality rate.91011) Furthermore, psychological distress can change during the course of cancer treatment after initial diagnosis.1213) Therefore, comprehensive and periodic patient assessment focused on psychological as well as physical aspects should be incorporated into the treatment process for cancer patients, particularly those with advanced or metastatic cancer. Although there are many studies on psychological distress and QOL in cancer patients,1415161718) there are few studies on the relation between psychological distress and QOL in patients with advanced or metastatic gastrointestinal cancer.1920)
The objective of this study was to assess the factors associated with QOL in Korean patients with advanced gastrointestinal cancer requiring palliative chemotherapy at initial diagnosis of advanced cancer and during the palliative treatment process, and to determine whether anxiety and depression are predictive of QOL.
All consecutive eligible patients treated at the Department of Gastrointestinal Medical Oncology in Chungbuk National University Hospital were considered for this observational prospective study. The following inclusion criteria were applied: age>18 years; histologically confirmed advanced gastrointestinal cancer including esophagus, gastric, colorectal, liver, pancreas, and bile duct cancers; scheduled to receive palliative chemotherapy; Eastern Cooperative Oncology Group (ECOG) performance-status (PS) score≤2 (on a 5-point scale, with 0 indicating no symptoms and higher scores indicating increasing disability)21); life expectancy≥3 months; ability to understand the Korean language; and normal cognitive function. Patients were excluded if they had a terminal condition, cognitive impairment, history of a psychological disorder or substance abuse, or a severe comorbid illness. This observational study was reviewed and approved by the Institutional Review Board of Chungbuk National University Hospital. Written informed consent was obtained from all participants prior to enrollment in the study.
Sociodemographic data (marital status, education level, employment status, and monthly income) were obtained from standardized questions. Clinical data (age, sex, ECOG PS, comorbid disease, primary site of tumor, and type of anticancer therapy) were obtained from a physician's assessment and the patient's medical record.
QOL was evaluated using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30), Korean version 3.22) EORTC QLQ-C30 is organized into three subscales; global health status, function scales, and symptom scales.23) Higher scores for global health status and on the function scales indicate better overall QOL and level of functioning, and higher scores on the symptom scales indicate a higher level of symptomatology and worse QOL. Yun et al.22) confirmed the validity of EORTC QLQ-C30 Korean version and reported that Cronbach's α coefficients ranged from 0.60 to 0.89. In the present study, Cronbach's α coefficients were 0.89 for global health status, 0.91 for functional scales, and 0.81 for symptom scales, respectively. The use of EORTC QLQ-C30 in this study was approved by the EORTC-QLQ Study group.
Emotional functioning including anxiety or depression and pain in EORTC QLQ-C30 were included in the analysis of the functional and symptom scales of EORTC QLQ-C30 for overall assessment of QOL. Anxiety, depression, and pain were assessed by each assessment measure with proven reliability and validity. Anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale (HADS), Korean version.24) The HADS is a 14-item questionnaire consisting of two subscales, anxiety and depression, each including seven items. Each item is scored on a four-point scale ranging from zero to three, and subscale scores range from zero, indicating no distress, to 21, indicating maximum distress. A score≥8 on either of the two HADS subscales is considered to be clinically significant. Oh et al.24) demonstrated the validity of measures of anxiety and depression and reported that Cronbach's α coefficients in HADS anxiety and depression were 0.89 and 0.86, respectively. In the present study, Cronbach's α coefficients of HADS anxiety and depression were 0.88 and 0.82, respectively. The use of HADS Korean version in this study was approved by the Korean Translater. Pain intensity was evaluated using a numeric rating scale (NRS) ranging from zero (no pain) to 10 (worst pain imaginable).
QOL, pain, anxiety, and depression were evaluated at initial diagnosis of advanced gastrointestinal cancer (study enrollment) and during the palliative treatment process (3 months after study enrollment). The reason for setting 3 months as the point of the palliative treatment process is that most of the patients will have active anticancer treatment at the 3 months after initial diagnosis even considering the gastrointestinal cancer with poor prognosis such as liver, pancreas, and bile duct cancers.
Data are summarized as frequencies and percentages for categorical variables, and mean and standard deviation (SD) for continuous variables. QOL, pain, anxiety, and depression were compared between baseline and 3 month time points using independent sample t-tests.
At both baseline and 3 months, QOL was compared across patients grouped according to age, sex, marital status, education level, employment status, monthly income, ECOG PS, comorbid disease, primary site of tumor, and type of anticancer therapy using an independent sample t-test or one-way analysis of variance (ANOVA). Statistically significant ANOVA results were followed up using Duncan's post-hoc analysis. Relations between continuous variables (pain, anxiety, and depression) and QOL were quantified using Pearson's correlation coefficients.
At both baseline and 3 months, factors affecting QOL were identified using a multiple regression analysis based on a stepwise method. Prior to the regression analysis, we performed an independent validation of the homoscedasticity and normality of errors. This was followed by the validation of multicolinearity.
p<.05 was considered statistically significant. All statistical analyses were performed using SPSS for Windows software, version 18.0 (SPSS Inc., Chicago, IL).
Of the 129 patients screened between July 2012 and June 2014, 120 were included in the study. Nine patients were excluded because of cognitive impairment (n=2), refusal to participate (n=4), or inability to fill in the questionnaires (n=3)(Fig. 1). Of the 120 patients included in the study, 100 patients (83.3%) completed the anxiety, depression, and QOL evaluations at baseline and at 3 month time points, and 20 had incomplete data at the 3 month time point because they were lost to follow-up (n=11) or died before the 3 month assessment (n=9)(Fig. 1). The sociodemographic and clinical characteristics of the 120 patients included in the study are shown in Table 1. There was no significant change in QOL (EORTC QLQ-C30 score), pain (NRS score), anxiety (HADS anxiety score), or depression (HADS depression score) between baseline and 3 months (Table 2).
QOL according to sociodemographic and clinical factors at baseline and 3 months are listed in Table 3. At baseline, the EORTC QLQ-C30 global health status score was greater in employed patients than in unemployed patients, greater in patients with low ECOG PS scores than in patients with high ECOG PS scores. The EORTC QLQ-C30 symptom scales score was greater (indicating worse QOL) in patients aged<50 years than in patients aged 50~59 years, 60~69 years, and>70 years, and greater in patients aged 50~59 years than in patients aged 60~69 years and>70 years.
At 3 months, the EORTC QLQ-C30 global health status score was greater in patients with low ECOG PS scores than in patients with high ECOG PS score. The EORTC QLQ-C30 functional scales score was greater in patients who had not completed high school than in patients who had completed high school, and greater in patients with low ECOG PS scores than in patients with with high ECOG PS score. The EORTC QLQ-C30 symptom scales score was greater (indicating worse QOL) in patients who had completed high school than in patients who had not completed high school, lower (indicating better QOL) in in patients with low ECOG PS score than in patients with high ECOG PS scores.
At both baseline and 3 months there were negative correlations between EORTC QLQ-C30 global health status score and pain, anxiety, and depression; negative correlations between EORTC QLQ-C30 functional scales score and pain, anxiety, and depression; and positive correlations between EORTC QLQ-C30 symptom scales score and pain, anxiety, and depression (Table 4). The strength of the correlations between QOL and pain, anxiety, and depression were greater at 3 months than at baseline. At both time points, the strength of the correlation between QOL and depression was stronger than that between QOL and anxiety, with the exception of the symptoms dimension of QOL (EORTC QLQ-C30 symptom scales score) at baseline.
The predictive factors for QOL at baseline and at 3 months are listed in Table 5. At baseline, depression, ECOG PS, and anxiety accounted for 55.0% (adjusted R2 = 0.55, p<.001) of the variance in EORTC QLQ-C30 global health status score, depression accounted for 22.0% (adjusted R2 = 0.22, p<.001) of the variance in EORTC QLQ-C30 functional scales score, and anxiety accounted for 19.0% (adjusted R2 = 0.19, p<.001) of the variance in EORTC QLQ-C30 symptom scales score. At 3 months, depression, pain, and ECOG PS accounted for 72.0% (adjusted R2 = 0.72, p<.001) of the variance in EORTC QLQ-C30 global health status score, 76.0% (adjusted R2 = 0.76, p<.001) of the variance in EORTC QLQ-C30 functional scales score, and 74.0% (adjusted R2 = 0.74, p<.001) of the variance in EORTC QLQ-C30 symptom scales score. Depression and ECOG PS were significant predictors of QOL at baseline and 3 months, anxiety was a significant predictor of QOL at baseline, and pain was a significant predictor of QOL at 3 months.
No previous study has reported relationship between psychological distress and QOL in Korean patients with advanced gastrointestinal cancer, despite the incidence of gastrointestinal cancer has a higher incidence than other cancers in Korea. The aim of this prospective study was to assess the factors that are associated with QOL in Korean patients with advanced gastrointestinal cancer requiring palliative chemotherapy at initial diagnosis of advanced cancer and during the palliative treatment process and to determine whether anxiety and depression are predictive of QOL. In the present study, we found that anxiety, depression, ECOG PS, and pain were predictive of QOL, and the factors that were predictive of QOL were different at initial diagnosis of advanced cancer and after 3 months of treatment. Depression and PS were significant predictive factors of QOL at both baseline and 3 months, and anxiety and pain were significant predictive factors of QOL at baseline and 3 months, respectively. Our results provide baseline data for the development of psychological interventions adapted according to the time since initial diagnosis for the improvement of QOL in patients with advanced gastrointestinal cancer.
There is accumulating evidence of an association between psychological distress and QOL in cancer patients.1415161718) In Korea, the prevalence of anxiety or depression in cancer patients has been estimated as 28.8~56.5% when all stages and cancer types are considered.2526) However, there are few studies that have focused on anxiety and depression in patients with gastrointestinal cancer, or that have reported an association between psychological distress and QOL in patients with gastrointestinal cancer.192027) Alacacioglu et al20) reported that anxiety and depression were strongly associated with poor QOL in Turkish colorectal cancer patients, and Tsunoda et al27) reported that depression had a stronger impact than anxiety on the global QOL of colorectal cancer patients. In the present study, participants reported a moderate level of QOL. This is consistent with a previous study of patients with advanced colorectal cancer,20) but symptom QOL in our study was much lower (indicating better QOL) than that reported in a study of patients with metastatic gastrointestincal cancer,23) because esophageal cancer was the most common tumor type23) and patients with esophageal cancer commonly experience symptomatic problems caused by dysphagia, nausea, vomiting, and pain. The objective of this study was to assess the factors associated with QOL at initial diagnosis of advanced cancer and during the palliative treatment process. There was no significant change in QOL between baseline and 3 months in patients with advanced gastrointestinal cancer. It is possible that only patients with advanced or metastatic gastrointestinal cancer requiring palliative chemotherapy were included, but all included patients had good PS (ECOG PS≤2). However, psychologic symptoms, such as anxiety or depression, were significant predictors of QOL in patients with advanced gastrointestinal caner. Furthermore, psychological symptoms associated with QOL changed before and after treatment; anxiety was a significant predictive factor of QOL at baseline and depression was a significant predictive factor of QOL at both baseline and 3 months. In the present study, 37 (30.8%) of the 120 patients at baseline were identified as having anxiety or depression according to HADS, which is consistent the results reported in a previous study of Korean cancer patients at all cancer stages and with all cancer types.26) The mean HADS anxiety score decreased from 2.60 points at baseline to 2.30 points at 3 months, and the mean HADS depression score increased from 5.49 points at baseline to 5.86 points at 3 months, although there were no statistically significant changes in anxiety or depression between baseline and at 3 months. Gil et al12) also reported that anxiety decreased and depression increased from pre to post cancer treatment. The emotional impact of learning of a cancer diagnosis may explain the high level of anxiety immediately after diagnosis. With the progression of the treatment, patients may become more aware of the significance of the cancer diagnosis, and this is likely followed by an increased awareness of the effects of the disease on all aspects of life. This may lead to patients becoming more depressed. In patients with advanced cancer requiring palliative therapy, there is a gradual increase in uncomfortable somatic symptoms, and this is accompanied by difficulties associated with treatment procedures such as chemotherapy. This may also contribute to an increase in depressive symptoms.
Pain is one of the most common symptoms in cancer patients, and significantly affects the functional status and QOL of cancer patients. 2829) In a previous study of patients with gastrointestinal cancer, the presence of cancer pain was associated with a higher prevalence of depression and a lower QOL (global health status), role and emotional functioning.18) The incidence of cancer pain in the present study was much lower (38% at baseline and 54.2% at 3 months) than in previous studies conducted on patients with metastatic cancer (64~90%).28) Many previous studies have included inpatients with metastatic cancer or patients with progressive cancer after the initial diagnosis of metastatic cancer.28) By contrast, the current study focused on patients who were initially diagnosed with advanced gastrointestinal cancer at the outpatient clinic. In the current study, cancer pain was significant predictive factor of all dimensions of QOL after 3 months of treatment, i.e., at the time when there is a gradual increase in pain associated with the adverse effects of cancer therapy, such as chemotherapy or progression of the cancer itself.
The present study has several limitations. First, we did not perform a comprehensive analysis of the effects of palliative therapy on somatic, emotional, social, and spiritual symptoms, even though the adverse effects of cancer treatment and the response to cancer treatment have been associated with QOL.30) Second, we measured QOL only at the initial diagnosis of advanced gastrointestinal cancer and 3 months after the initial diagnosis. Other social and economic problems may reduce QOL after diagnosis of advanced cancer and during the course of the disease, and severe psychological distress and physical symptoms caused by the uncontrolled growth of the tumor may affect QOL in terminal cancer patients who have stopped active chemotherapy. We suggest that all cancer patients should be screened periodically for QOL and psychological symptoms as part of standard cancer care from initial diagnosis to death. Lastly, the number of patients recruited to the study was small, meaning there was insufficient statistical power for detailed subgroup analyses, and all patients were from a single institution, making it difficult to generalize our results.
In conclusion, depression and performance status are key factors that need to be improved to efficiently improve QOL in patients with advanced gastrointestinal cancer, regardless of the time relative to diagnosis. Anxiety should be monitored in the early stage after a diagnosis of advanced cancer, and pain should be monitored in the later stage where there is a gradual increase in pain with the progression of the cancer and the adverse effects of cancer therapy become apparent. Therefore, systematic assessments and comprehensive psychological interventions for anxiety and depression are required periodically for patients with advanced gastrointestinal cancer.
Figures and Tables
Table 1
Sociodemographic and Clinical Characteristics (N=120)

Table 2
Quality of Life, Anxiety, and Depression at Baseline and 3 Months
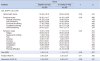
Table 3
Quality of Life according to Sociodemographic and Clinical Characteristics

Table 4
Correlations between Quality of Life and Pain, Anxiety, and Depression

Table 5
Predictive Factors for Quality of Life

References
1. National Cancer Institute. Surveillance, epidemiology, and end results program. Assessed November 28, 2016. http://seer.cancer.gov.
2. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Community of population-based regional cancer registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48(2):436–450.

3. Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee JK, et al. Prediction of cancer incidence and mortality in Korea, 2016. Cancer Res Treat. 2016; 48(2):451–457.

4. National Cancer Information Center. Cancer Statistics in Korea. Assessed November 28, 2016. http://www.cancer.go.kr.
5. Polanski J, Jankowska-Polanska B, Rosinczuk J, Chabowski M, Szymanska-Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther. 2016; 9:1023–1028.
6. van Nieuwenhuizen AJ, Buffart LM, Brug J, Leemans CR, Verdonck-de Leeuw IM. The association between health related quality of life and survival in patients with head and neck cancer: a systematic review. Oral Oncol. 2015; 51(1):1–11.

7. Svensson H, Hatschek T, Johansson H, Einbeigi Z, Brandberg Y. Health-related quality of life as prognostic factor for response, progression-free survival, and survival in women with metastatic breast cancer. Med Oncol. 2012; 29(2):432–438.

8. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010; 363(8):733–742.

9. Pirl WF, Greer JA, Traeger L, Jackson V, Lennes IT, Gallagher ER, et al. Depression and survival in metastatic non-small-cell lung cancer: effects of early palliative care. J Clin Oncol. 2012; 30(12):1310–1315.

10. Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011; 29(4):413–420.

11. Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010; 40(11):1797–1810.

12. Gil F, Costa G, Hilker I, Benito L. First anxiety, afterwards depression: psychological distress in cancer patients at diagnosis and after medical treatment. Stress Health. 2012; 28(5):362–367.

13. Annunziata MA, Muzzatti B, Bidoli E. Psychological distress and needs of cancer patients: a prospective comparison between the diagnostic and the therapeutic phase. Support Care Cancer. 2010; 19(2):291–295.

14. Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, et al. American Society of Clinical Oncology. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014; 32(15):1605–1619.

15. Bužgová R, Jarošová D, Hajnová E. Assessing anxiety and depression with respect to the quality of life in cancer inpatients receiving palliative care. Eur J Oncol Nurs. 2015; 19(6):667–672.

16. Montazeri A. Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 2009; 7:102.

17. Slovacek L, Slovackova B, Slanska I, Petera J, Priester P. Quality of life and depression among metastatic breast cancer patients. Med Oncol. 2010; 27(3):958–959.

18. Tavoli A, Montazeri A, Roshan R, Tavoli Z, Melyani M. Depression and quality of life in cancer patients with and without pain: the role of pain beliefs. BMC Cancer. 2008; 8:177.

19. Matsushita T, Matsushima E, Maruyama M. Psychological state, quality of life, and coping style in patients with digestive cancer. Gen Hosp Psychiatry. 2005; 27(2):125–132.

20. Alacacioglu A, Binicier O, Gungor O, Oztop I, Dirioz M, Yilmaz U. Quality of life, anxiety, and depression in Turkish colorectal cancer patients. Support Care Cancer. 2010; 18(4):417–421.

21. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5(6):649–655.

22. Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, et al. Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res. 2004; 13(4):863–868.

23. Fard JH, Janbabaei G. Quality of life and its related factors among Iranian patients with metastatic gastrointestinal tract cancer: a cross-sectional study. Indian J Palliat Care. 2014; 20(3):215–219.

24. Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for Koreans: A comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999; 38(2):289–296.
25. Shim EJ, Shin YW, Jeon HJ, Hahm BJ. Distress and its correlates in Korean cancer patients: pilot use of the distress thermometer and the problem list. Psychooncology. 2008; 17(6):548–555.

26. Kim SJ, Rha SY, Song SK, Namkoong K, Chung HC, Yoon SH, et al. Prevalence and associated factors of psychological distress among Korean cancer patients. Gen Hosp Psychiatry. 2011; 33(3):246–252.

27. Tsunoda A, Nakao K, Hiratsuka K, Yasuda N, Shibusawa M, Kusano M. Anxiety, depression and quality of life in colorectal cancer patients. Int J Clin Oncol. 2005; 10(6):411–417.

28. van den Beuken-van Everdingen MH, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007; 18(9):1437–1449.

29. Green CR, Hart-Johnson T, Loeffler DR. Cancer-related chronic pain: examining quality of life in diverse cancer survivors. Cancer. 2011; 117(9):1994–2003.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download