Abstract
Purpose
Dysregulation of adipokines caused by excess adipose tissue has been implicated in the development of obesity-related metabolic diseases. This study evaluated the effects of mugwort (Artemisia princeps Pampanini) ethanol extract on lipid metabolic changes, insulin resistance, adipokine balance, and body fat reduction in obese rats.
Methods
Male Sprague-Dawley rats were fed either a control diet (NC), high-fat diet (HF, 40% kcal from fat), or high-fat diet with 1% mugwort extract (HFM) for 6 weeks.
Results
Epididymal and retroperitoneal fat mass increased in the HF group compared with the NC group, and epididymal fat mass was reduced in the HFM group (p < 0.05). No difference was observed in serum levels of total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) among the groups. However, triglyceride (TG), TG/HDL-C ratio, and TC/HDL-C ratio increased in the HF group and significantly decreased in the HFM group. TG and TC levels in the liver were significantly higher in the HF group, whereas these levels were significantly reduced in the HFM group. HF rats had lower insulin sensitivity as indicated by increased homeostasis model assessment of the insulin resistance (HOMA-IR) value. HOMA-IR values significantly decreased in the HFM group. Adiponectin levels were higher in NC rats, and their leptin and PAI-1 levels were lower. Relative balance of adipokines was reversed in the HF group, with lower adiponectin levels but higher leptin and PAI-1 levels. In contrast, the HFM group maintained balance of adiponectin/leptin and adiponectin/PAI-1 levels similar to NC by reducing leptin and PAI-1 levels.
Go to : 
References
1. Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012; 42(6):563–570.

2. Grundy SM. Obesity, metabolic syndrome, and cardiovascular disease. J Clin Endocrinol Metab. 2004; 89(6):2595–2600.

3. Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am J Physiol Endocrinol Metab. 2007; 293(6):E1736–E1745.

4. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011; 11(2):85–97. .5. Fain JN. Release of inflammatory mediators by human adipose tissue is enhanced in obesity and primarily by the nonfat cells: a review. Mediators Inflamm 2010;2010: 513948.

6. Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Pract Res Clin Endocrinol Metab. 2002; 16(4):653–666.

7. Ruige JB, Dekker JM, Blum WF, Stehouwer CD, Nijpels G, Mooy J, Kostense PJ, Bouter LM, Heine RJ. Leptin and variables of body adiposity, energy balance, and insulin resistance in a population-based study. The Hoorn Study. Diabetes Care. 1999; 22(7):1097–1104.

8. Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998; 395(6704):763–770.

9. Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013; 417:80–84.

10. Kim DL, Kim NH, Shin DH, Kim SG, Choi KM, Kim JK, Shin C, Lee SG, Baik SH, Choi DS. Plasma leptin concentration, obesity, and insulin resistance in healthy Korean population. J Korean Diabetes. 2002; 26(2):100–111.
11. Park MY, Ahn SA, Cho WK, Cho KS, Park SH, Hahn SH, Jung MH, Suh BK. Serum leptin, adiponectin and resistin levels in obese children and their correlations with insulin resistance. Korean J Pediatr. 2009; 52(7):766–771.

12. Hayes MG, Sobel BE, Taatjes DJ, Rincon M, Schneider DJ. Attenuation of migration of vascular smooth muscle cells by over expression of plasminogen activator inhibitor type-1. J Am Coll Cardiol. 2002; 39:203A.
13. De Taeye B, Smith LH, Vaughan DE. Plasminogen activator inhib-itor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol. 2005; 5(2):149–154.
14. Lee MY, Kim JH. Comparison of serum insulin, leptin, adiponectin and high sensitivity c-reactive protein levels according to body mass index and their associations in adult women. Korean J Community Nutr. 2011; 16(1):126–135.

15. Kim M, Kim J, Bae W, Kim S, Lee Y, Na W, Sohn C. Relationship between nutrients intakes, dietary quality, and serum concentrations of inflammatory markers in metabolic syndrome patients. Korean J Community Nutr. 2011; 16(1):51–61.

16. Lee MY, Kim JH. Association of serum lipids and dietary intakes with serum adiponectin level in overweight and obese Korean women. Korean J Community Nutr. 2010; 15(1):27–35.
17. Nam SM, Ham SS, Oh DH, Kang IJ, Lee SY, Chung CK. Effects of Artemisia iwayomogi Kitamura ethanol extract on lowering serum and liver lipids in rats. J Korean Soc Food Sci Nutr. 1998; 27(2):338–343.
18. Lim SS, Kim MH, Lee JH. Effect of Artemisia princeps var orientalis and circium japonicum var ussuriense on liver function, body lipid, and bile acid of hyperlipidemic rat. Korean J Nutr. 1997; 30(7):797–802.
19. Kang JR, Lee SJ, Hwang CR, Kim IS, Sung NJ. Effect of black garlic and Gaeddongssuk (Artemisia annua L.) extracts on the lipid profile and hepatic antioxidant enzyme activities of exercised rats. J Korean Soc Food Sci Nutr. 2013; 42(6):869–876.

20. Park JC, Yu YB, Lee JH, Kim NJ. Studies on the chemical components and biological activities of edible plants in Korea(VI): anti-inflammatory and anlagesic effects of Cedrela sinensis, Oenanthe javanica and Artemisia princeps var. orientalis. J Korean Soc Food Nutr. 1994; 23(1):116–119.
21. Kim HT, Kim DD, Ku SK, Kim JW, Jang KH, Oh TH, Lee KW. Anti-obestic effects of Artemisiae capillaris herba, Artemisia capillaris stem aqueous extracts on the high fat diet supplied mice. J Vet Clin. 2010; 27(4):348–365.
22. Kang YJ, Jung UJ, Lee MK, Kim HJ, Jeon SM, Park YB, Chung HG, Baek NI, Lee KT, Jeong TS, Choi MS. Eupatilin, isolated from Artemisia princeps Pampanini, enhances hepatic glucose metabolism and pancreatic beta-cell function in type 2 diabetic mice. Diabetes Res Clin Pract. 2008; 82(1):25–32.
23. Park JS. Effects of ethanolic extracts of Artemisia princeps Pam-panini cv. Sajabal in high-fat diet-induced obese mice [dissertation]. Daejeon: Chungnam National University;2009.
24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28(7):412–419.
25. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226(1):497–509.

26. Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002; 13(1):51–59.

27. Sypniewska G. Proinflammatory and prothrombotic factors and metabolic syndrome. EJIFCC. 2007; 18(1):
28. Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007; 115(4):450–458.
29. Goldberg IJ. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis. J Lipid Res. 1996; 37(4):693–707.

30. Rizzo M, Pernice V, Frasheri A, Di Lorenzo G, Rini GB, Spinas GA, Berneis K. Small, dense low-density lipoproteins (LDL) are predictors of cardio- and cerebrovascular events in subjects with the metabolic syndrome. Clin Endocrinol (Oxf). 2009; 70(6):870–875.

31. Engfeldt P, Arner P. Lipolysis in human adipocytes, effects of cell size, age and of regional differences. Horm Metab Res Suppl. 1988; 19:26–29.
32. Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis. 1996; 124(Suppl):S1–S9.

33. Marsh JB. Lipoprotein metabolism in obesity and diabetes: insights from stable isotope kinetic studies in humans. Nutr Rev. 2003; 61(11):363–375.

34. Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008; 134(5):1369–1375.

35. Björntorp P. Abdominal obesity and the development of noninsulindependent diabetes mellitus. Diabetes Metab Rev. 1988; 4(6):615–622.

36. Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983; 72(5):1737–1747.

37. Yamamoto N, Kanemoto Y, Ueda M, Kawasaki K, Fukuda I, Ash-ida H. Anti-obesity and antidiabetic effects of ethanol extract of Artemisia princeps in C57BL/6 mice fed a high-fat diet. Food Funct. 2011; 2(1):45–52.

38. Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014; 15(4):6184–6223.

39. Lefebvre AM, Laville M, Vega N, Riou JP, van Gaal L, Auwerx J, Vidal H. Depot-specific differences in adipose tissue gene expression in lean and obese subjects. Diabetes. 1998; 47(1):98–103.

40. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996; 334(5):292–295.

41. Bastard JP, Piéroni L, Hainque B. Relationship between plasma plasminogen activator inhibitor 1 and insulin resistance. Diabetes Metab Res Rev. 2000; 16(3):192–201.

42. Ma LJ, Mao SL, Taylor KL, Kanjanabuch T, Guan Y, Zhang Y, Brown NJ, Swift LL, McGuinness OP, Wasserman DH, Vaughan DE, Fogo AB. Prevention of obesity and insulin resistance in mice lacking plasminogen activator inhibitor 1. Diabetes. 2004; 53(2):336–346.

43. Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8(11):1288–1295.

44. Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999; 257(1):79–83.

45. Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002; 43(9):1363–1379.

47. Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002; 147(2):173–180.

48. Kim JY, Shin HW, Jeong IK, Cho SW, Min SJ, Lee SJ, Park CY, Oh KW, Hong EG, Kim HK, Kim DM, Yu JM, Ihm SH, Choi MG, Yoo HJ, Park SW. The relationship of adiponectin, leptin and ghrelin to insulin resistance and cardiovascular risk factors in human obesity. Korean J Med. 2005; 69(6):631–641.
Go to : 
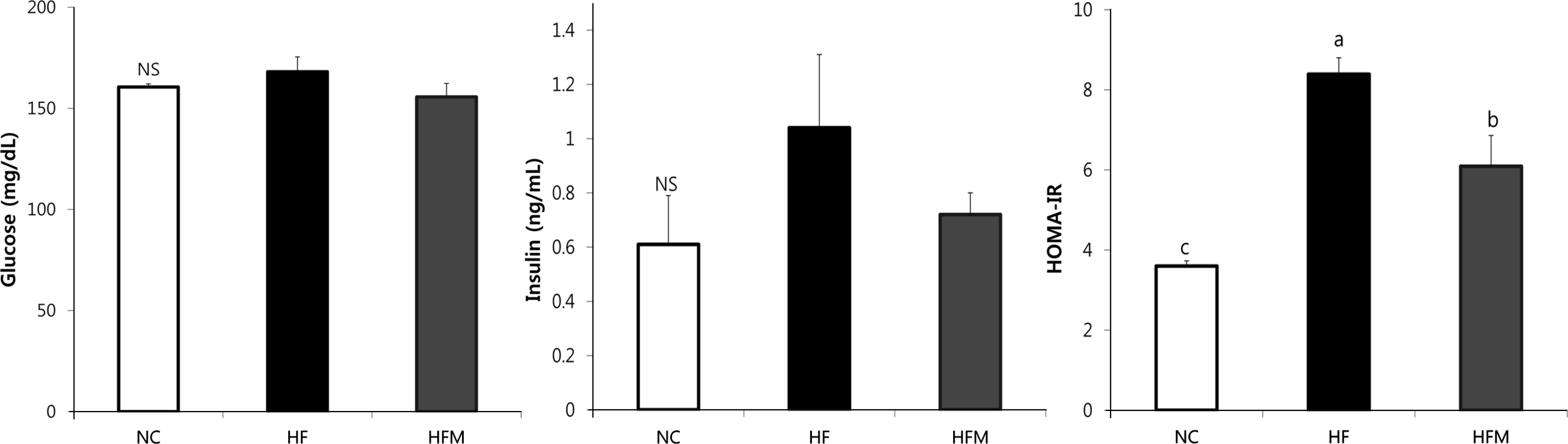 | Fig. 1.Effect of mugwort ethanol extract on plasma levels of glucose, insulin and HOMA-IR. Data are presented as mean ± SEM. Bars with uncommon letters are significantly different at p < 0.05 using one-way ANOVA followed by Duncan's multiple range test. NC: control diet, HF: high fat diet with no supplement, HFM: high fat diet with 1% mugwort ethanol extract, HOMA-IR: homeostasis model assessment of insulin resistance |
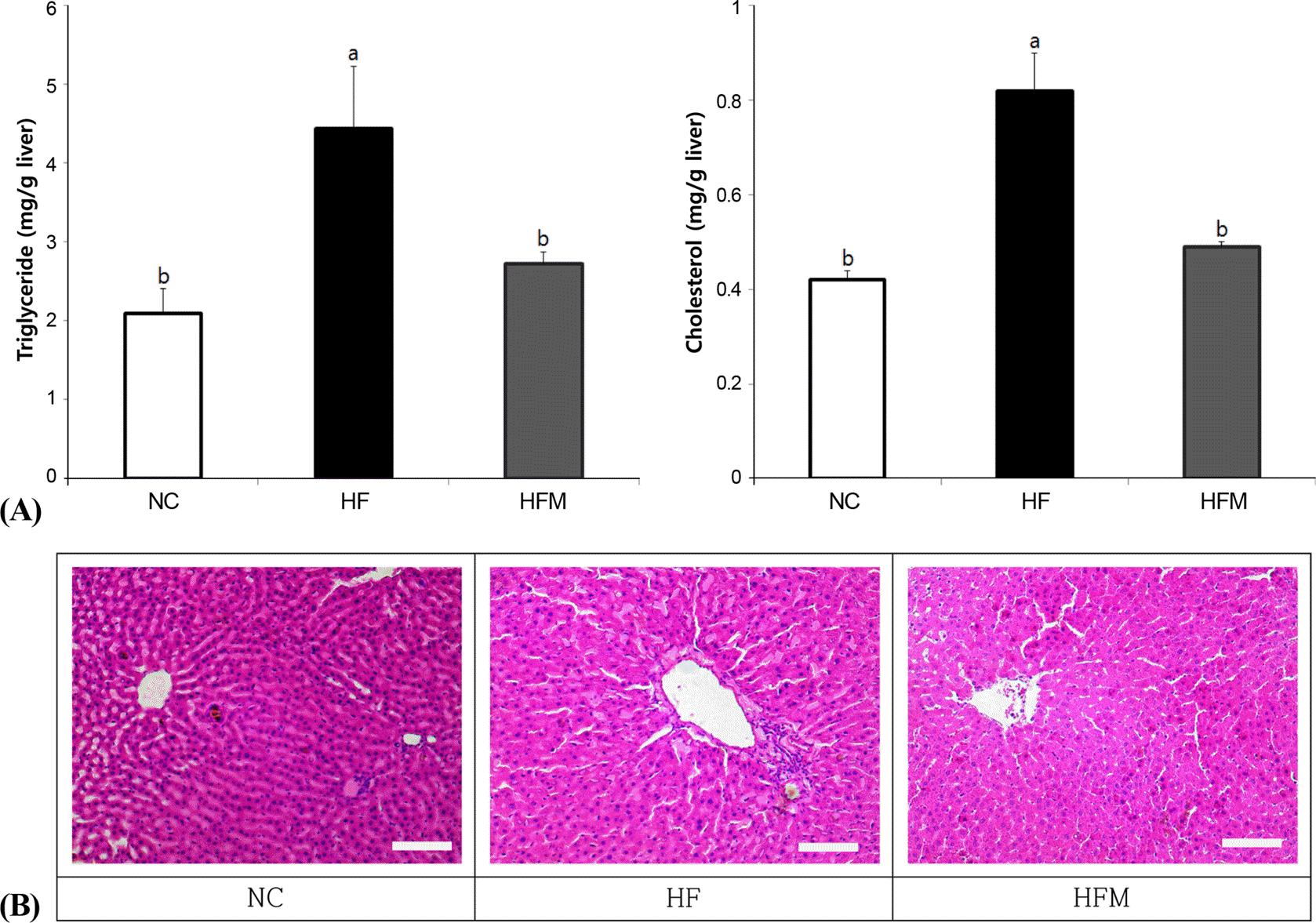 | Fig. 2.Effect of mugwort ethanol extract on lipid accumulation in liver (A) Triglyceride and cholesterol levels in liver, (B) Histopathological observation of liver. Data are presented as mean ± SEM. Bars with uncommon letters are significantly different at p < 0.05 using one-way ANOVA followed by Duncan's multiple range test. NC: control diet, HF: high fat diet with no supplement, HFM: high fat diet with 1% mugwort ethanol extract. Scale bars = 100 µm |
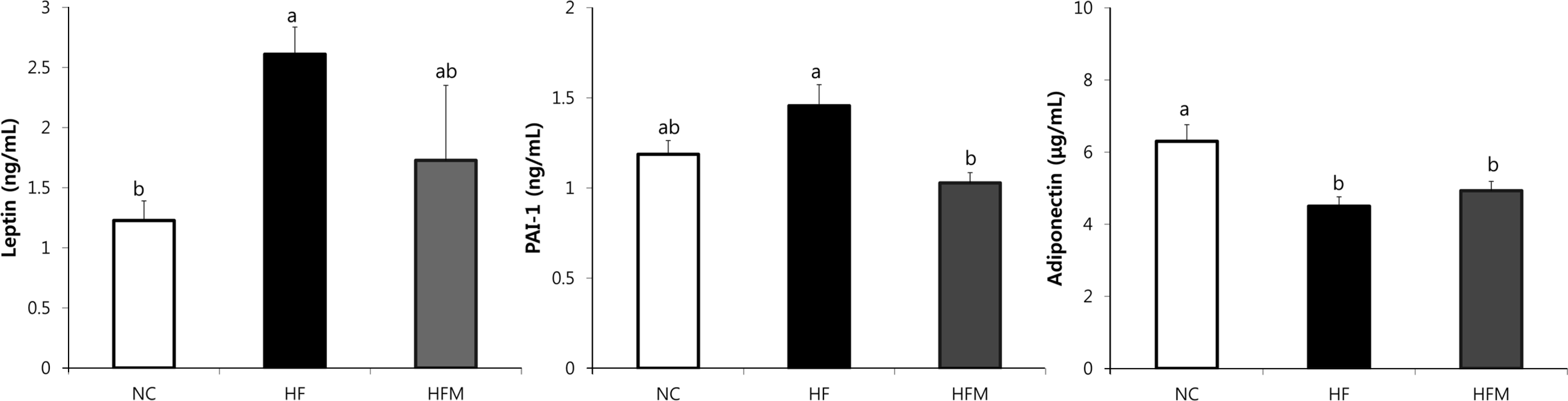 | Fig. 3.Effect of mugwort ethanol extract on plasma levels of leptin, PAI-1 and adiponectin. Data are presented as mean ± SEM. Bars with uncommon letters are significantly different at p < 0.05 using one-way ANOVA followed by Duncan's multiple range test. NC: control diet, HF: high fat diet with no supplement, HFM: high fat diet with 1% mugwort ethanol extract |
Table 1.
Composition of experimental diets (g/kg)
Table 2.
Effect of mugwort ethanol extract on change in body, liver and adipose tissue weight
Table 3.
Effect of mugwort ethanol extract on plasma levels of triglyceride, total cholesterol and HDL-C




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download