Abstract
Purpose
Aloe-emodin (AE), an ingredient of aloe, is known to exhibit anti-inflammatory activities. However, little is known about the underlying molecular mechanisms of its inflammatory modulatory activity in vitro. In the present study, we investigated the anti-inflammatory potential of AE using Pam3CSK4-stimulated macrophages.
Methods
RAW 264.7 macrophages were treated with AE (0~20 mM) for 1 h, followed by treatment with Pam3CSK4 for 1 h. After incubation, mRNA expression levels of cytokines were measured. The effect of AE on TLR2-related molecules was also investigated in Pam3CSK4-stimulated RAW 264.7 macrophages.
Results
AE attenuated Pam3CSK4-stimulated expression of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) in RAW 264.7 macrophages. Two concentrations of AE (10 µM and 20 µM) effectively reduced mRNA expression of TLR2 by 41.18% and 54.43%, respectively, compared to that in control cells (p < 0.05). AE also decreased nuclear factor-kappa B (NF-κB) activation and mitogen-activated protein kinase (MAPK) phosphorylation. Phosphorylation levels of ERK1/2, p38, and JNK were markedly reduced by 20 µM AE. In particular, AE decreased phosphorylation of ERK in a dose-dependent manner in Pam3CSK4-stimulated RAW 264.7 macrophages.
Aloe leaves are used in the treatment of several medical conditions, including burns, cancer, and inflammatory bowel disease.123 Aloe-emodin (AE), a major anthraquinone present in aloe, exhibits antibacterial, antiviral, anti-inflammatory, and anticancer effects.456 The levels of AE and aloin, a C-glycoside derivative of AE, range from 0.1% to 21.5% of dry weight in leaf exudates of 68 Aloe species.78 Previous studies have used in vitro and in vivo models to study the anti-inflammatory activity of emodin. Park et al. reported that AE dose-dependently inhibited the levels of nitric oxide (NO) and prostaglandin E2 (PGE2) by blocking the mRNA expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in LPS-stimulated macrophages.9 Yin et al. reported that emodin ameliorated lung injury via the inhibition of cytokine production and inhibition of the p38 mitogen-activated protein kinase (MAPK) pathway in an animal model.10 Iwanowycz et al. have reported that emodin bidirectionally regulates macrophage phagocytosis and migration by blocking the nuclear factor kappa B (NF-κB)/interferon regulatory factor 5 (IRF5)/signal transducer and activator of transcription 1 (STAT1) and interferon regulator factor 4 (IRF4)/signal transducer and activator of transcription 6 (STAT6) signaling pathways.11 However, few studies have investigated the biological activity of AE, and its exact mechanisms have not been fully elucidated.
Chronic inflammation is associated with several diseases. Since macrophages are implicated in the initiation of inflammatory responses, they play an important role in inflammatory diseases.1213 Therefore, the inhibition of macrophage-mediated inflammatory responses is a useful therapeutic approach against several inflammatory diseases. Toll-like receptors (TLRs) are pattern recognition receptors that recognize several pathogen-associated molecular pattern (PAMP) molecules involved in pathogenic invasions.1415 PAMP-recognizing TLRs stimulate signaling pathways that involve NF-κB and MAPKs.
The aim of this study was to investigate the anti-inflammatory activity of AE against synthetic triacylated lipoprotein Pam3CSK4-stimulated RAW 264.7 macrophages.
AE and Pam3CSK4 were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against extracellular signal-regulated kinase (ERK), p38, c-Jun N-terminal kinase (JNK), phospho (p)-ERK (Thr202/Tyr204), p-p38 (Thr180/Tyr182), and p-JNK (Thr183/Tyr185) were purchased from Cell Signaling Technology (Beverly, MA, USA). All other chemicals were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
The mouse macrophage cell line (RAW 264.7) was purchased from American Type Culture Collection (Manassas, VA, USA). Cells were grown in DMEM supplemented with 10% heat-inactivated fetal bovine serum, and 1% penicillin/streptomycin. Cells were incubated at 37℃, 5% CO2 for 5~7 days until monolayers of macrophages were observed.
Total RNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. Real-time quantitative polymerase chain reaction (PCR) was performed using a QuantitectTM SYBR Green PCR kit (QuantitectTM SYBR Green PCR, Qiagen, CA, USA). The specific primer sets were as follows: 5'-AACATCCAACCTTCCCAAACG-3'/5'-CTCTTAACCCCCGAATCCCAG-3' for the tumor necrosis factor alpha (TNF-α) gene, 5'-TCACCTCTTCAGAACGAATTGACA-3'/5'-AGTGCCTCTTGCTGCTTTCAC-3' for the interleukin 6 (IL-6) gene, 5'-ATTGGGATCATCTTGCTGGT-3'/5'-CCTGCTGTTCACAGTTGCC-3' for the interleukin 1β (IL-1β) gene, and 5'-GAGCGCAAGTACTCTGTGTG-3'/5'-CGGACTCATCGTACTCCTG-3' for the β-actin gene used as an endogenous control. The relative mRNA expression levels of cytokines were normalized to that β-actin using the ΔΔCT method.16
To determine the NF-κB activity, the nuclears of macrophages were extracted using a Nuclear Extract kit (Active Motif, Carlsbad, CA, USA) and analyzed using a PathScan Total NF-κB p65 assay kit (Cell Signaling Technology, MA, USA) according to the manufacturer's instructions.
Whole cell extract was prepared by suspending in an extraction lysis buffer and the cellular proteins were extracted with Laemmli sample buffer. Proteins were separated on 10% sodium dodecyl sulfate (SDS) polyacrylamide gels electrophoresis and then transferred to polyvinylidene fluoride (PVDF) membrane. The membranes were incubated with 5% nonfat dry milk, followed by incubation with appropriate primary antibodies in 5% nonfat dry milk in 0.05% Tris-buffered saline with Tween 20 (TBS-T) at 4℃ overnight. The membranes were washed three times with TBS-T and then incubated for 1 h at room temperature with appropriate horseradish peroxidase-conjugated secondary antibodies. The membranes were visualized by chemiluminescence (ECL) and the density of the blots was quantified by ChemiDoc MP imaging system (Bio-Rad, Hercules, CA, USA).
To evaluate whether AE can inhibit the gene expression of cytokines, we measured the TNF-α mRNA level in AE-pretreated RAW 264.7 macrophages after their stimulation with Pam3CSK4. We observed that Pam3CSK4 upregulated the TNF-α mRNA expression, but the overexpression was inhibited by both 10 µM and 20 µM AE (Fig. 1).
Next, we measured the mRNA expression levels of proinflammatory cytokines in Pam3CSK4-stimulated RAW 264.7 macrophages by real-time PCR. Our results showed that AE at concentrations of 1-20 µM significantly inhibited the mRNA expression levels of all cytokines studied (Fig. 2; p < 0.05). AE significantly decreased the mRNA expression levels of IL-6 and IL-1β in a dose-dependent manner in this cell model (Fig. 2B, C; p < 0.05). Among the three proinflam-matory cytokines, TNF-α was most effectively blocked by AE treatment of macrophages (Fig. 2A; p < 0.05).
Next, we evaluated the inhibitory effect of AE on the Pam3CSK4-stimulated TLR2 mRNA expression in macrophages. AE significantly inhibited the Pam3CSK4-stimulated TLR2 mRNA overexpression at both 10 µM and 20 µM concentrations (Fig. 3; p < 0.05). Pretreatment with 10 µM and 20 µM AE decreased the mRNA expression of TLR2 by 41.18% and 54.43%, respectively, compared to that in the control cells (Fig. 3; p < 0.05).
To understand the molecular mechanism(s) underlying the TLR2-blocking effect, NF-κB and MAPK activation were examined in RAW 264.7 macrophages. Our results showed that the NF-κB activity was dramatically upregulated by the Pam3CSK4 treatment compared with the activity in the Pam3CSK4-stimulated control group (Fig. 4A). AE at 10 µM and 20 µM reduced the NF-κB activity by 26.83% and 46.34%, respectively, compared with that in the Pam3CSK4-stimulated control cells (Fig. 4A; p < 0.05).
Next, we examined the MAPK phosphorylation in Pam3CSK4-stimulated RAW 264.7 macrophages. The phosphorylation of ERK1/2, p38, and JNK was reduced by 20 µM AE (Fig. 4B). In particular, AE decreased the ERK phosphorylation in a dose-dependent manner in the Pam3CSK4-stimulated RAW 264.7 macrophages. These results indicated that the anti-inflammatory efficacy of AE on RAW 264.7 is associated with the inactivation of NF-κ+B, as well as with blocking of MAPK phosphorylation.
TLRs are pattern recognition molecules, which represent major components of the innate immune response. Their specific signaling pathways are associated with several inflammatory diseases.17 In particular, TLR2 is widely distributed on the surface of several types of immune cells, including macrophages, dendritic cells, and mast cells.181920 The receptor is the main sensor for PAMP recognition of gram-positive bacteria. Pam3CSK4 binds to the host TLR2 and leads to an inflammatory reaction.21 In this study, we investigated whether AE can inhibit the Pam3CSK4-induced, TLR2-regulated signaling in RAW 264.7 macrophages.
To test the anti-inflammatory potential of AE, the mRNA expression level of the inflammatory marker TNF-α was measured in AE-pretreated RAW 264.7 macrophages after stimulation with Pam3CSK4. Our results showed that AE downregulated the TNF-α mRNA expression at concentrations of 10 µM and 20 µM (Fig. 1). Our results also showed that AE at concentrations of 5-20 µM effectively suppressed the mRNA levels for all the cytokines (TNF-α, IL-6, and IL-1β) in RAW 264.7 macrophages (Fig. 2). These data indicated that AE protects against the Pam3CSK4-induced inflammatory response in macrophages. Related in vivo studies have shown that emodin significantly ameliorated inflammatory responses through inhibition of cytokine overproduction. Nemmar et al. have suggested that emodin administration protects from diesel exhaust particle-induced lung inflammation via inhibition of TNF-α, IL-6, and IL-1β in mice.22 According to Han et al., the treatment with emodin decreased the IL-1β secretion by blocking the activation of the NLRP3 inflammasome in alipopolysaccharide-induced endotoxin mouse model.23
To understand the mechanism of AE in regulating Pam3CSK4-induced cytokine gene expression, we examined the TLR2 mRNA expression in Pam3CSK4-stimulated macrophages. We found that AE effectively reduced upregulated expression of TLR2 (Fig. 3). These results indicate that AE down-regulates TLR2-mediated cytokine induction. There are a few studies evaluating the inhibitory effect of AE on TLR2-mediated signaling pathways. Li et al. reported that emodin from the Chinese herb Radix et Rhizoma Rhe significantly decreased the expression of cytokine genes by blocking TLR2 signaling pathways in rat kidney epithelial cells.24
The effect of AE in TLR-2 pathways may have associated with the inhibition of the NF-κB signaling pathway, which is the major transcription pathway for inflammatory responses.25 The results showed that NF-κB activity was dramatically enhanced by Pam3CSK4 treatment compared with the activity in the Pam3CSK4-negative control group; however, this enhancement of the activity was effectively alleviated in the 10 µM and 20 µM AE-treated groups (Fig. 4A). A previous study indicated that AE effectively decreased the activation of p38 and NF-κB in a concanavalin A-induced animal hepatitis model.26 Although there are few data on AE, several in vivo studies have reported that emodin effectively ameliorates inflammatory diseases via inactivation of NF-κB.2728 This evidence indicates that AE is a potential anti-inflammatory agent that inhibits both NF-κB activation and gene expression of proinflammatory cytokines.
Because the activation of NF-κB as well as MAPK pathways are implicated in TLR2 signaling, we evaluated the protein levels of p-ERK1/2, p-p38, and p-JNK in Pam3CSK4-stimulated macrophages.
Pam3CSK4 stimulation upregulated the phosphorylations of the MAPKs in RAW 264.7 macrophages. We have also observed that 20 µM AE treatment prevented an increased expression of MAPKs (Fig. 4B), which is consistent with previous observations showing that emodin suppreses the activation of p38 and ERK1/2.2930 These results demonstrated that AE shows an anti-inflammatory action that is mediated by a TLR2-dependent MAPK signaling pathway in macrophages.
Figures and Tables
 | Fig. 1Effect of AE on Pam3CSK4-induced TNF-α mRNA expression in RAW 264.7 macrophages. RAW 264.7 macrophages were pretreated with 0~20 µM AE for 1 h. The cells were further stimulated with Pam3CSK4 (1 µg/mL). After 1 h, the TNF-α mRNA expression was analyzed in an agarose gel. |
 | Fig. 2Effect of AE on the Pam3CSK4-induced TNF-α (A), IL-6 (B), and IL-1β (C) mRNA expression in RAW 264.7 macrophages. RAW 264.7 macrophages were pretreated with 0~20 µM AE for 1 h and further stimulated with Pam3CSK4 (1 µg/mL). After 1 h, TNF-α, IL-6, and IL-1β mRNA expression was determined. The data are expressed as a fold induction compared with the vehicle-treated cells. The values are the mean ± SD (n = 3). *Significantly different from the Pam3CSK4-stimulated control (p < 0.05). |
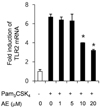 | Fig. 3Effect of AE on the Pam3CSK4-induced TLR2 mRNA expression in RAW 264.7 macrophages. RAW 264.7 macrophages were pretreated with 0~20 µM AE for 1 h and further stimulated with Pam3CSK4 (1 µg/mL). After 1 h, TLR2 mRNA expression was determined. The data are expressed as a fold induction relative to the vehicle-treated cells. The values are the mean ± SD (n = 4). *Significantly different from the Pam3CSK4-stimulated control, p < 0.05. |
 | Fig. 4Effect of AE on the NF-κB activation (A) and MAPK expression (B) in RAW 264.7 macrophages. RAW 264.7 macrophages were pretreated with 0~20 µM AE for 1 h and further stimulated with Pam3CSK4 (1 µg/mL). After 1 h, NF-κB activity was determined (A), and cell lysates were analyzed by MAPK immunoblotting (B). The NF-κB activity data are expressed as % activation compared with the vehicle-treated cells. The values are the mean ± SD (n = 4). *Significantly different from the Pam3CSK4-stimulatedcontrol, p < 0.05. |
References
1. Farzadinia P, Jofreh N, Khatamsaz S, Movahed A, Akbarzadeh S, Mohammadi M, Bargahi A. Anti-inflammatory and wound healing activities of aloe vera, honey and milk ointment on second-degree burns in rats. Int J Low Extrem Wounds. 2016; 15(3):241–247.

2. Al-Oqail MM, El-Shaibany A, Al-Jassas E, Al-Sheddi ES, Al-Massarani SM, Farshori NN. In vitro anti-proliferative activities of Aloe perryi flowers extract on human liver, colon, breast, lung, prostate and epithelial cancer cell lines. Pak J Pharm Sci. 2016; 29:2 Suppl. 723–729.
3. Park MY, Kwon HJ, Sung MK. Dietary aloin, aloesin, or aloe-gel exerts anti-inflammatory activity in a rat colitis model. Life Sci. 2011; 88(11-12):486–492.

4. Andersen DO, Weber ND, Wood SG, Hughes BG, Murray BK, North JA. In vitro virucidal activity of selected anthraquinones and anthraquinone derivatives. Antiviral Res. 1991; 16(2):185–196.

5. Arosio B, Gagliano N, Fusaro LM, Parmeggiani L, Tagliabue J, Galetti P, De Castri D, Moscheni C, Annoni G. Aloe-Emodin quinone pretreatment reduces acute liver injury induced by carbon tetrachloride. Pharmacol Toxicol. 2000; 87(5):229–233.

6. Esmat AY, Tomasetto C, Rio MC. Cytotoxicity of a natural anthraquinone (Aloin) against human breast cancer cell lines with and without ErbB-2: topoisomerase IIalpha coamplification. Cancer Biol Ther. 2006; 5(1):97–103.

7. Groom QJ, Reynolds T. Barbaloin in aloe species. Planta Med. 1987; 53(4):345–348.
8. van Wyk BE, van Rheede van Oudtshoorn MC, Smith GF. Geographical variation in the major compounds of Aloe ferox leaf exudate. Planta Med. 1995; 61(3):250–253.
9. Yin JT, Wan B, Liu DD, Wan SX, Fu HY, Wan Y, Zhang H, Chen Y. Emodin alleviates lung injury in rats with sepsis. J Surg Res. 2016; 202(2):308–314.

10. Iwanowycz S, Wang J, Altomare D, Hui Y, Fan D. Emodin bidirectionally modulates macrophage polarization and epigenetically regulates macrophage memory. J Biol Chem. 2016; 291(22):11491–11503.

12. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008; 8(12):958–969.

13. Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000; 20(5):393–405.

14. Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003; 300(5625):1524–1525.

15. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25(4):402–408.
16. Salvador B, Arranz A, Francisco S, Córdoba L, Punzón C, Llamas MÁ, Fresno M. Modulation of endothelial function by Toll like receptors. Pharmacol Res. 2016; 108(9):46–56.

17. McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003; 170(4):1625–1629.

18. Redecke V, Häcker H, Datta SK, Fermin A, Pitha PM, Broide DH, Raz E. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J Immunol. 2004; 172(5):2739–2743.

19. Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005; 17(1):1–14.
21. Nemmar A, Al-Salam S, Yuvaraju P, Beegam S, Ali BH. Emodin mitigates diesel exhaust particles-induced increase in airway resistance, inflammation and oxidative stress in mice. Respir Physiol Neurobiol. 2015; 215(5):51–57.

22. Han JW, Shim DW, Shin WY, Heo KH, Kwak SB, Sim EJ, Jeong JH, Kang TB, Lee KH. Anti-inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation. Int J Mol Sci. 2015; 16(4):8102–8109.
23. Gambhir V, Yildiz C, Mulder R, Siddiqui S, Guzzo C, Szewczuk M, Gee K, Basta S. The TLR2 agonists lipoteichoic acid and Pam3CSK4 induce greater pro-inflammatory responses than inactivated Mycobacterium butyricum. Cell Immunol. 2012; 280(1):101–107.

24. Li Y, Xiong W, Yang J, Zhong J, Zhang L, Zheng J, Liu H, Zhang Q, Ouyang X, Lei L, Yu X. Attenuation of inflammation by emodin in lipopolysaccharide-induced acute kidney injury via inhibition of toll-like receptor 2 signal pathway. Iran J Kidney Dis. 2015; 9(3):202–208.
25. Dahiya Y, Pandey RK, Sodhi A. Nod2 downregulates TLR2/1 mediated IL1β gene expression in mouse peritoneal macrophages. PLoS One. 2011; 6(11):e27828.

26. Xue J, Chen F, Wang J, Wu S, Zheng M, Zhu H, Liu Y, He J, Chen Z. Emodin protects against concanavalin A-induced hepatitis in mice through inhibiting activation of the p38 MAPK-NF-κB signaling pathway. Cell Physiol Biochem. 2015; 35(4):1557–1570.

27. Yao WY, Zhou YF, Qian AH, Zhang YP, Qiao MM, Zhai ZK, Yuan YZ, Yang SL. Emodin has a protective effect in cases of severe acute pancreatitis via inhibition of nuclear factor κB activation resulting in antioxidation. Mol Med Rep. 2015; 11(2):1416–1420.
28. Xiao M, Zhu T, Zhang W, Wang T, Shen YC, Wan QF, Wen FQ. Emodin ameliorates LPS-induced acute lung injury, involving the inactivation of NF-κB in mice. Int J Mol Sci. 2014; 15(11):19355–19368.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download