Abstract
Purpose
Diabetic complications are a major concern to manage progression of diabetes. Production of advanced glycation end products (AGEs) due to high blood glucose is one of the mechanisms leading to diabetic complications. Multiple pharmacologic AGE inhibitory agents are currently under development, but clinical applications are still limited due to safety issues. Thus, it is necessary to identify a safe anti-glycation agent. It is known that burdock roots have antioxidant, anti-inflammatory, and anti-cancer activities. The objective of the present study was to investigate the inhibitory role of burdock roots on the formation of high glucose-induced glycation of bovine serum albumin (BSA).
Methods
In this study, glycation of BSA by glucose, galactose, or fructose at 37 ° C for 3 weeks was assessed based on levels of α-dicarbonyl compounds (early-stage glycation products), fructosamine (intermediate products of glycation), and fluorescent AGEs (late-stage glycation products). In order to compare the inhibitory actions of burdock root extract in AGE formation, aminoguanidine (AG), a pharmacological AGE inhibitor, was used as a positive control.
Results
BSA glycation by glucose, fructose, and galatose was dose- and time-dependently produced. Burdock root extract at a concentration of 4 mg/mL almost completely inhibited glucose-induced BSA glycation. The results demonstrate that burdock root extract inhibited AGE formation with an IC50 value of 1.534 mg/mL, and inhibitory activity was found to be more effective than the standard anti-glycation agent aminoguanidine. This study identified a novel function of burdock root as a potential anti-glycation agent.
Go to : 
References
1. Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, Khunti K. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and metaanalysis. Diabetes Care. 2014; 37(4):922–933.
2. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014; 103(2):137–149.

3. Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Lim S, Choi SH, Jang MJ, Kim Y, Oh K, Kim DJ, Cha BY. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J. 2013; 37(5):349–357.

4. Jeon JY, Kim DJ, Ko SH, Kwon HS, Lim S, Choi SH, Kim CS, An JH, Kim NH, Won JC, Kim JH, Cha BY, Song KH. Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association. Current status of glycemic control of patients with diabetes in Korea: the fifth Korea national health and nutrition examination survey. Diabetes Metab J. 2014; 38(3):197–203.

5. Bogdanov VY, Østerud B. Cardiovascular complications of diabetes mellitus: the tissue factor perspective. Thromb Res. 2010; 125(2):112–118.

6. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008; 26(2):77–82.

7. Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. UKPDS GROUP. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 2003; 63(1):225–232.

8. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002; 287(19):2570–2581.
9. Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004; 164(13):1422–1426.
10. Ceriello A, Testa R. Antioxidant anti-inflammatory treatment in type 2 diabetes. Diabetes Care. 2009; 32(Suppl 2):S232–S236.

11. Cooper ME, Bonnet F, Oldfield M, Jandeleit-Dahm K. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens. 2001; 14(5 Pt 1):475–486.

12. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010; 107(9):1058–1070.

13. Gugliucci A. Glycation as the glucose link to diabetic complications. J Am Osteopath Assoc. 2000; 100(10):621–634.
14. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014; 18(1):1–14.

15. Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001; 36(9):1527–1537.

16. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014; 14(1):453–464.

17. Schleicher E, Friess U. Oxidative stress, AGE, and atherosclerosis. Kidney Int Suppl. 2007; 106:S17–S26.

18. Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991; 40(4):405–412.

19. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002; 23(5):599–622.

20. Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein crosslinking. Science. 1986; 232(4758):1629–1632.

21. Thornalley PJ. Use of aminoguanidine (Pimagedine) to prevent the formation of advanced glycation endproducts. Arch Biochem Biophys. 2003; 419(1):31–40.

22. Viberti G, Slama G, Pozza G, Czyzyk A, Bilous RW, Gries A, Keen H, Fuller JH, Menzinger G. Steering Committee. Safety Committee. Early closure of European Pimagedine trial. Lancet. 1997; 350(9072):214–215.
23. Pari L, Saravanan G. Antidiabetic effect of cogent db, a herbal drug in alloxan-induced diabetes mellitus. Comp Biochem Physiol C Toxicol Pharmacol. 2002; 131(1):19–25.

24. Zhao F, Wang L, Liu K. In vitro anti-inflammatory effects of arcti-genin, a lignan from Arctium lappa L., through inhibition on iNOS pathway. J Ethnopharmacol. 2009; 122(3):457–462.

25. Maruta Y, Kawabata J, Niki R. Antioxidative caffeoylquinic acid derivatives in the roots of burdock (Arctium lappa L.). J Agric Food Chem. 1995; 43(10):2592–2595.

26. Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SM, Leung GP, Yu PH, Chan SW. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology. 2011; 19(5):245–254.

27. Kim M, Lee Y, Sohn H. Anti-thrombosis and antioxidative activity of the root of Arctium lappa L. Korean J Food Preserv. 2014; 21(5):727–734.
28. de Almeida AB, Luiz-Ferreira A, Cola M, Di Pietro Magri L, Batista LM, de Paiva JA, Trigo JR, Souza-Brito AR. Anti-ulcero-genic mechanisms of the sesquiterpene lactone onopordopicrin-enriched fraction from Arctium lappa L. (Asteraceae): role of somatostatin, gastrin, and endogenous sulfhydryls and nitric oxide. J Med Food. 2012; 15(4):378–383.

29. Sugiura Y, Torii T, Matsuda K, Yamada Y. Anti-allergic effects of extracts from commercial products of cooked burdock. Food Sci Technol Res. 2009; 15(4):423–426.

30. Wu J, Hsieh C, Wang H, Chen H. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009; 113:78–84.

31. Wells-Knecht KJ, Zyzak DV, Litchfield JE, Thorpe SR, Baynes JW. Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry. 1995; 34(11):3702–3709.
32. Yeboah FK, Alli I, Yaylayan VA. Reactivities of D-glucose and D-fructose during glycation of bovine serum albumin. J Agric Food Chem. 1999; 47(8):3164–3172.

33. Münch G, Taneli Y, Schraven E, Schindler U, Schinzel R, Palm D, Riederer P. The cognition-enhancing drug tenilsetam is an inhibitor of protein crosslinking by advanced glycosylation. J Neural Transm Park Dis Dement Sect. 1994; 8(3):193–208.

34. Ledesma-Osuna AI, Ramos-Clamont G, Vázquez-Moreno L. Characterization of bovine serum albumin glycated with glucose, galactose and lactose. Acta Biochim Pol. 2008; 55(3):491–497.

35. Kontogianni VG, Charisiadis P, Margianni E, Lamari FN, Geroth-anassis IP, Tzakos AG. Olive leaf extracts are a natural source of advanced glycation end product inhibitors. J Med Food. 2013; 16(9):817–822.

36. Hori M, Yagi M, Nomoto K, Shimode A, Ogura M, Yonei Y. Inhibition of advanced glycation end product formation by herbal teas and its relation to anti-skin aging. Anti Aging Med. 2012; 9(6):135–148.
37. Jariyapamornkoon N, Yibchok-anun S, Adisakwattana S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement Altern Med. 2013; 13(1):171–179.

38. Mesías M, Navarro M, Gökmen V, Morales FJ. Antiglycative effect of fruit and vegetable seed extracts: inhibition of AGE formation and carbonyl-trapping abilities. J Sci Food Agric. 2013; 93(8):2037–2044.

39. Predes FS, Ruiz AL, Carvalho JE, Foglio MA, Dolder H. Antioxidative and in vitro antiproliferative activity of Arctium lappa root extracts. BMC Complement Altern Med. 2011; 11(1):25–29.

Go to : 
 | Fig. 1.Measurement of advanced glycation end products (AGEs) production from incubation of bovine serum albumin (BSA) with three different sugars including glucose, fructose, and galactose. BSA (42 g/L) was incubated with 25 mM glucose, 25 mM fructose, or 25 mM galactose in 20 mM phosphate buffer saline (pH 7.4) at 37°C for 3 weeks. (A) In order to measure AGE production, fluorescence intensity was detected using excitation at 355 nm and emission at 460 nm every week. (B) Fructosamine formation was estimated using NBT test described in material and method section. All data were obtained from at least 3 independent experiments. The data represent the means ± SD (n = 6) and values are expressed as arbitrary unit (AU). For each incubation time, means sharing the same letter are not significantly different at 5% level. |
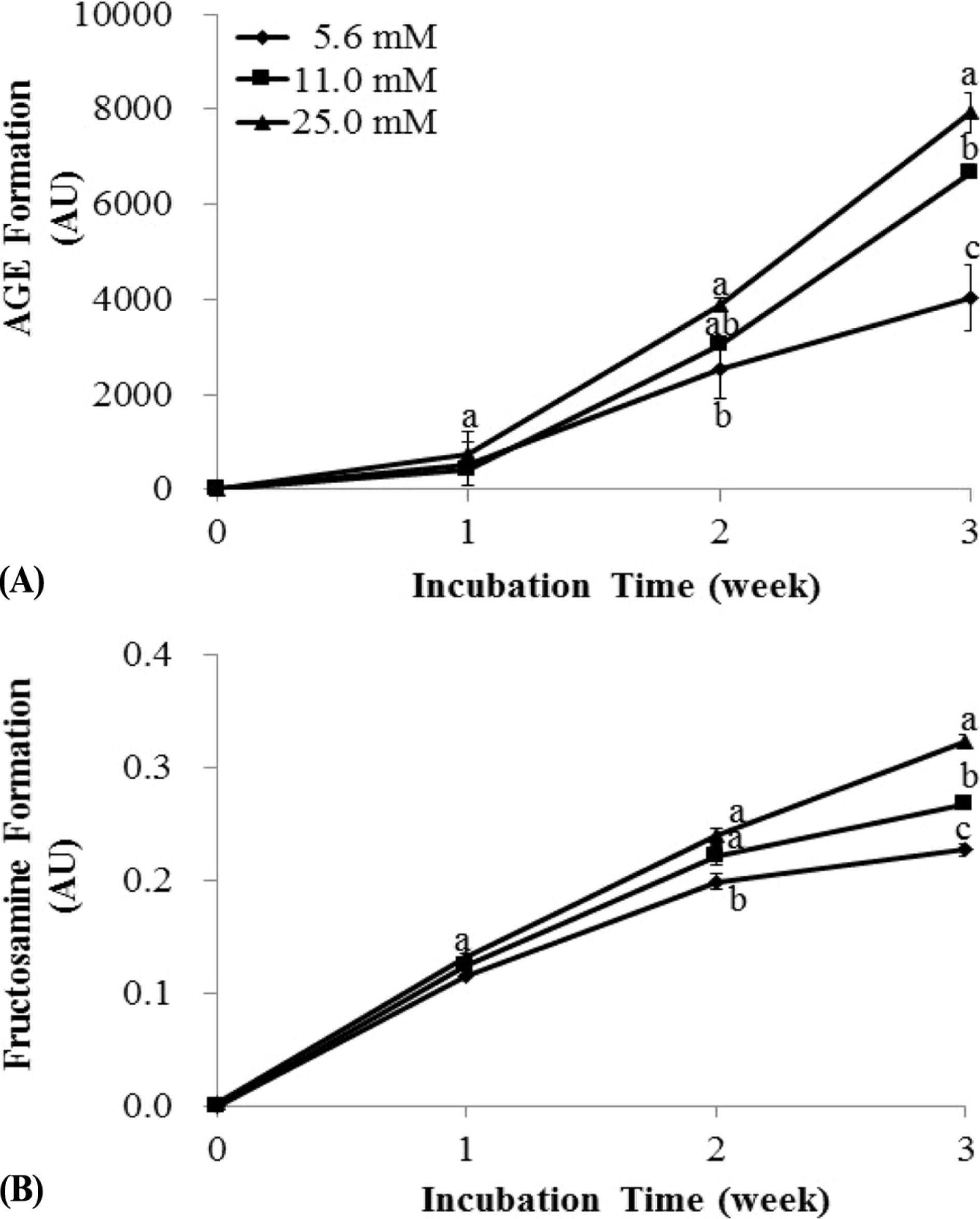 | Fig. 2.The effects of glucose concentration on advanced glycation end products (AGEs) generation in bovine serum albumin (BSA)/glucose system. In order to estimate the effect of glucose concentrations on formation of AGEs, BSA (42 g/L) was incubated with different concentrations (5.6, 11, and 25 mM) of glucose in 20 mM phosphate buffer saline (pH 7.4) at 37 ° C for 3 weeks. (A) Fluorescence intensity of AGEs was measured using excitation at 355 nm and emission at 460 nm each week. (B) Fructosamine formation was characterized by absorbance at 530 nm every week. The data represent the means ± SD (n = 6) and values are expressed as arbitrary unit (AU). For each incubation time, means sharing the same letter are not significantly different at 5% level. |
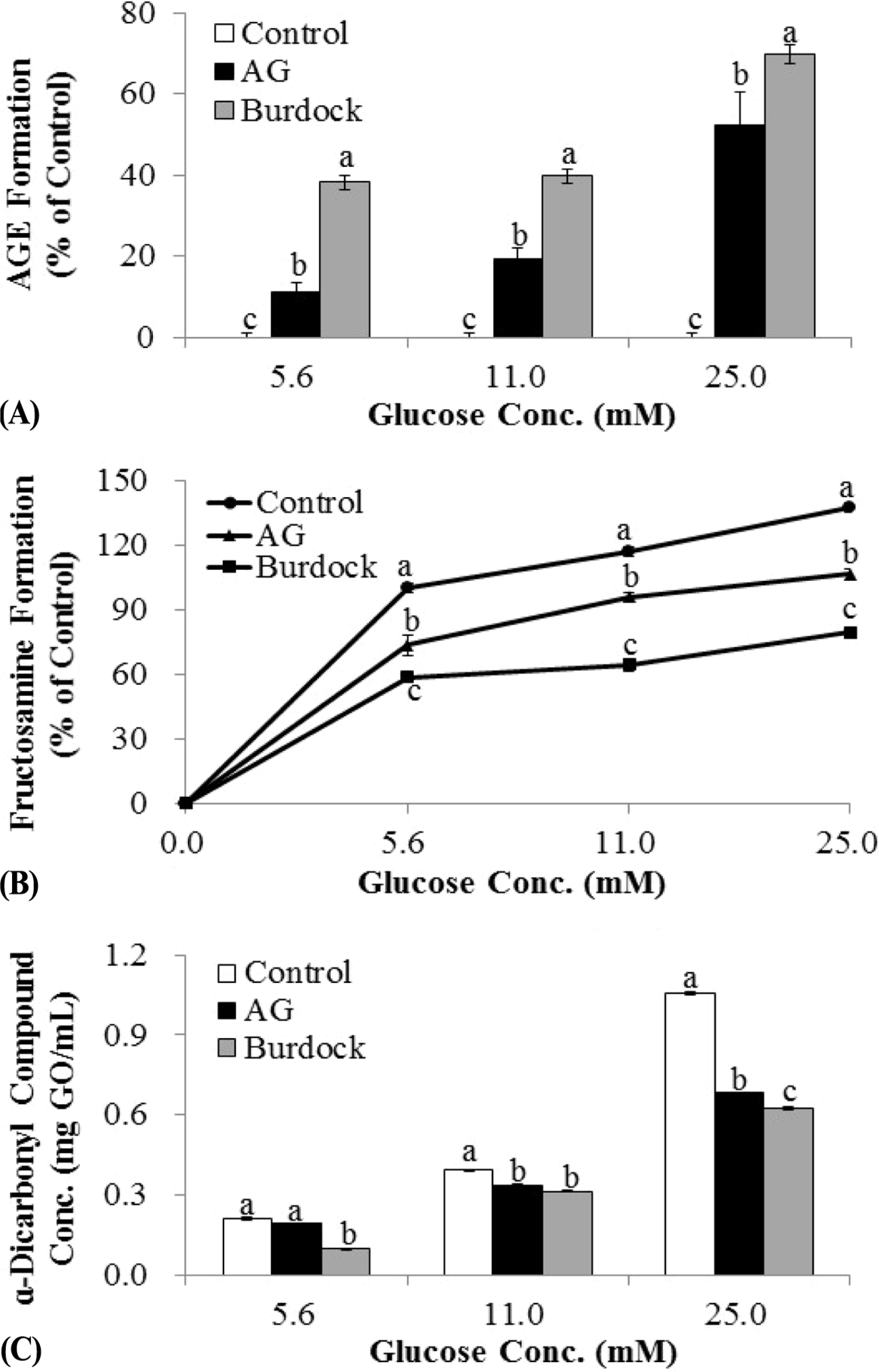 | Fig. 3.Inhibitory effect of burdock root extract on advanced glycation end products (AGEs) production from incubation of bovine serum albumin (BSA) with different concentrations of glucose. In order to examine the effect of burdock root extract on BSA (42 g/L) glycation with different concentrations (5.6, 11, and 25 mM) of glucose in 20 mM phosphate buffer saline (pH 7.4), 2 mg/mL concentration of burdock root extract was incubated with BSA-glucose system at 37°C for 3 weeks. Aminoguanidine (1 mM) was used as a positive control. Inhibition of AGE formation by burdock root extract was determined at 3 weeks of incubation by fluorimetric (A), NBT (B), and Girard-T (C) assays. All data were obtained from at least 3 independent experiments. The data represent the means ± SD (n = 6). For each glucose concentration, means sharing the same letter are not significantly different. |
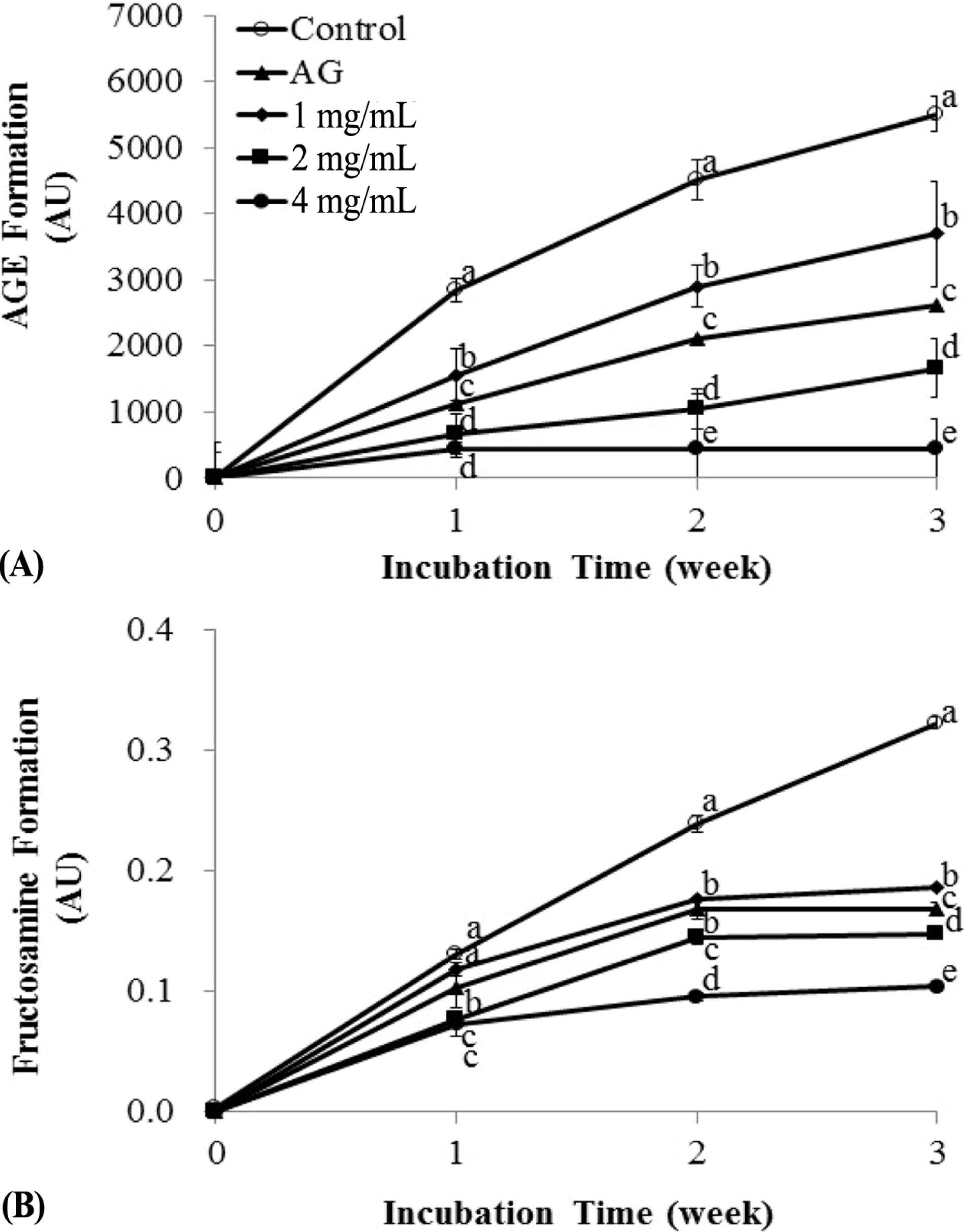 | Fig. 4.Anti-glycation activity of different concentrations of burdock root extract during 3 weeks of incubation. BSA (42 g/L) was incubated with 25 mM glucose in the absence (which is a control in the figure) and presence of different concentrations (1, 2, and 4 mg/mL) of burdock root extract at 37°C for 3 weeks. Aminoguanidine (1 mM) was used as a positive control. Fluorescence intensity (A) and fructosamine formation (B) were analyzed. All data were obtained from at least 3 independent experiments. For each incubation time, means sharing the same letter are not significantly different determined by Bonferroni's multiple comparison test (p > 0.05). |
Table. 1.
Inhibitory effect of burdock root extract on advanced glycation end products (AGEs) and fructosamine formation from incubation of bovine serum albumin (BSA) with three different monosaccharides at 3 weeks of incubation
|
Treatment |
Conc.1) |
AGEs |
Fructosamine |
|||
|---|---|---|---|---|---|---|
| Formation | Inhibition | Formation | Inhibition | |||
| (AU)2) | (%) | (AU) | (%) | |||
| Glucose | Control | 5,084 ± 1,821a | 0 ± 3.54 | 0.35 ± 0.01ab | 0 ± 2.51 | |
| Burdock | 2 mg/mL | 1,576 ± 1,204b | 69.76 ± 8.08 | 0.15 ± 0.01b | 58.08 ± 0.63 | |
| AG3) | 1 mM | 2,434 ± 112b | 52.52 ± 2.39 | 0.21 ± 0.02b | 39.94 ± 1.99 | |
| Fructose | Control | 6,527 ± 459a | 0 ± 1.24 | 0.52 ± 0.02a | 0 ± 3.45 | |
| Burdock | 2 mg/mL | 3,263 ± 263b | 50.13 ± 3.98 | 0.29 ± 0.02b | 44.45 ± 1.63 | |
| AG | 1 mM | 4,046 ± 202b | 38.88 ± 3.00 | 0.34 ± 0.09b | 34.11 ± 6.69 | |
| Galactose | Control | 5,755 ± 294a | 0 ± 2.41 | 0.42 ± 0.01a | 0 ± 5.64 | |
| Burdock | 2 mg/mL | 2,935 ± 1,092c | 49.93 ± 7.11 | 0.26 ± 0.03b | 39.42 ± 3.98 | |
| AG | 1 mM | 3,683 ± 1,145b | 36.76 ± 7.40 | 0.32 ± 0.08ab | 23.60 ± 5.77 | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download