Abstract
Purpose:
Poncirus trifoliata has been reported to have anti-inflammatory, antioxidant, and immune activities. However, its anti-obesity activity and the mechanism by which the water extract of dried, immature fruit of Poncirus trifoliata (PF-W) acts are not clear. This study suggests a potential mechanism associated with the anti-obesity activity of PF-W.
Methods:
We measured the effect of PF-W on lipoprotein lipase (LPL) regulation using enzyme-linked immunosorbent assay (ELISA) and an activity assay. The LPL regulation mechanism was examined by reverse transcription polymerase chain reaction (RT-PCR) to measure the mRNA expression of biomarkers related to protein transport and by western blot for analysis of the protein expression of the transcription factor CCAAT-enhancer-binding protein (C/EBPβ).
Results:
The total polyphenol and flavonoid content of PF-W was 52.15 ± 4.02 and 6.56 ± 0.47 mg/g, respectively. PF-W treatment decreased LPL content in media to 58 ± 5% of that in control adipocyte media, and increased LPL content to 117 ± 3.5% of that in control adipocytes, but did not affect the mRNA expression of LPL. PF-W also increased the mRNA expression of sortilin-related receptor (SorLA), a receptor that induces endocytosis and intracellular trafficking of LPL, in a concentration and time-dependent manner. Finally, cell fractionation revealed that PF-W treatment induced the expression of C/EBPβ, a SorLA transcription factor, in the nuclei of 3T3-L1 adipocytes.
Go to : 
REFERENCES
1.Spiegelman BM., Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996. 87(3):377–389.

3.Visscher TL., Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001. 22:355–375.

4.Chaput JP., St-Pierre S., Tremblay A. Currently available drugs for the treatment of obesity: Sibutramine and orlistat. Mini Rev Med Chem. 2007. 7(1):3–10.

5.Collins P., Williams G. Drug treatment of obesity: from past failures to future successes? Br J Clin Pharmacol. 2001. 51(1):13–25.

7.Vickers SP., Cheetham SC., Headland KR., Dickinson K., Grempler R., Mayoux E., Mark M., Klein T. Combination of the sodium-glucose cotransporter-2 inhibitor empagliflozin with orlistat or sibutramine further improves the body-weight reduction and glucose homeostasis of obese rats fed a cafeteria diet. Diabetes Metab Syndr Obes. 2014. 7:265–275.

8.Bae JS., Kim TH. Pancreatic lipase inhibitory and antioxidant activities of Zingiber officinale extracts. Korean J Food Preserv. 2011. 18(3):390–396.

9.Kumar P., Bhandari U., Jamadagni S. Fenugreek seed extract inhibit fat accumulation and ameliorates dyslipidemia in high fat diet-induced obese rats. Biomed Res Int. 2014. 2014:606021.

10.Wang H., Eckel RH. Lipoprotein lipase: from gene to obesity. Am J Physiol Endocrinol Metab. 2009. 297(2):E271–E288.

11.Braun JE., Severson DL. Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem J. 1992. 287(Pt 2):337–347.

12.Fernández-Sánchez A., Madrigal-Santillán E., Bautista M., Esquivel-Soto J., Morales-González A., Esquivel-Chirino C., Durante-Montiel I., Sánchez-Rivera G., Valadez-Vega C., Morales-González JA. Inflammation, oxidative stress, and obesity. Int J Mol Sci. 2011. 12(5):3117–3132.

13.Moreno DA., Ilic N., Poulev A., Brasaemle DL., Fried SK., Raskin I. Inhibitory effects of grape seed extract on lipases. Nutrition. 2003. 19(10):876–879.

14.Baek J., Lee J., Kim K., Kim T., Kim D., Kim C., Tsutomu K., Ochir S., Lee K., Park CH., Lee YJ., Choe M. Inhibitory effects of Capsicum annuum L. water extracts on lipoprotein lipase activity in 3T3-L1 cells. Nutr Res Pract. 2013. 7(2):96–102.
15.Lee E. Antihyperlipidemic and antioxidant effects of Poncirus trifoliata. Korean J Plant Resour. 2006. 19(2):273–276.
16.Shim WS., Back H., Seo EK., Lee HT., Shim CK. Long-term administration of an aqueous extract of dried, immature fruit of Poncirus trifoliata (L.) Raf. suppresses body weight gain in rats. J Ethno-pharmacol. 2009. 126(2):294–299.

17.Oh SD., Kim M., Min BI., Choi GS., Kim SK., Bae H., Kang C., Kim DG., Park BJ., Kim CK. Effect of achyranthes bidentata blume on 3T3-L1 adipogenesis and rats fed with a high-fat diet. Evid Based Complement Alternat Med. 2014. 2014:158018.
18.Lee SM., Kang YH., Kim DJ., Kim KK., Lim JG., Kim TW., Choe M. Comparison of antioxidant and α-glucosidase inhibition activities among water extracts and sugar immersion extracts of green pepper, purslane and shiitake. J East Asian Soc Diet Life. 2014. 24(1):101–108.

19.Feng Z., Hai-ning Y., Xiao-man C., Zun-chen W., Sheng-rong S., Das UN. Effect of yellow capsicum extract on proliferation and differentiation of 3T3-L1 preadipocytes. Nutrition. 2014. 30(3):319–325.

20.Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998. 56(11):317–333.

21.Lee GW., Park SM., Yoo YC., Cho YH. Effect of ponciri fructus extracts fermented with ganoderma lucidum on the collagen synthesis and expression of matrix metalloproteinase-1. Korean J Biotechnol Bioeng. 2013. 28(2):106–114.

22.Klinger SC., Glerup S., Raarup MK., Mari MC., Nyegaard M., Koster G., Prabakaran T., Nilsson SK., Kjaergaard MM., Bakke O., Nykjær A., Olivecrona G., Petersen CM., Nielsen MS. SorLA regulates the activity of lipoprotein lipase by intracellular trafficking. J Cell Sci. 2011. 124(Pt 7):1095–1105.

23.Hirayama S., Bujo H., Yamazaki H., Kanaki T., Takahashi K., Kobayashi J., Schneider WJ., Saito Y. Differential expression of LR11 during proliferation and differentiation of cultured neuroblastoma cells. Biochem Biophys Res Commun. 2000. 275(2):365–373.

24.Kim EY., Baik IH., Kim JH., Kim SR., Rhyu MR. Screening of the antioxidant activity of some medicinal plants. Korean J Food Sci Technol. 2004. 36(2):333–338.
25.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004. 79(5):727–747.

26.Kim EJ., Choi JY., Yu M., Kim MY., Lee S., Lee BH. Total polyphe-nols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J Food Sci Technol. 2012. 44(3):337–342.

27.Kumar S., Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013. 2013:162750.

28.Goldberg IJ., Merkel M. Lipoprotein lipase: physiology, biochemistry, and molecular biology. Front Biosci. 2001. 6:D388–D405.

29.Wu LG., Hamid E., Shin W., Chiang HC. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 2014. 76:301–331.

Go to : 
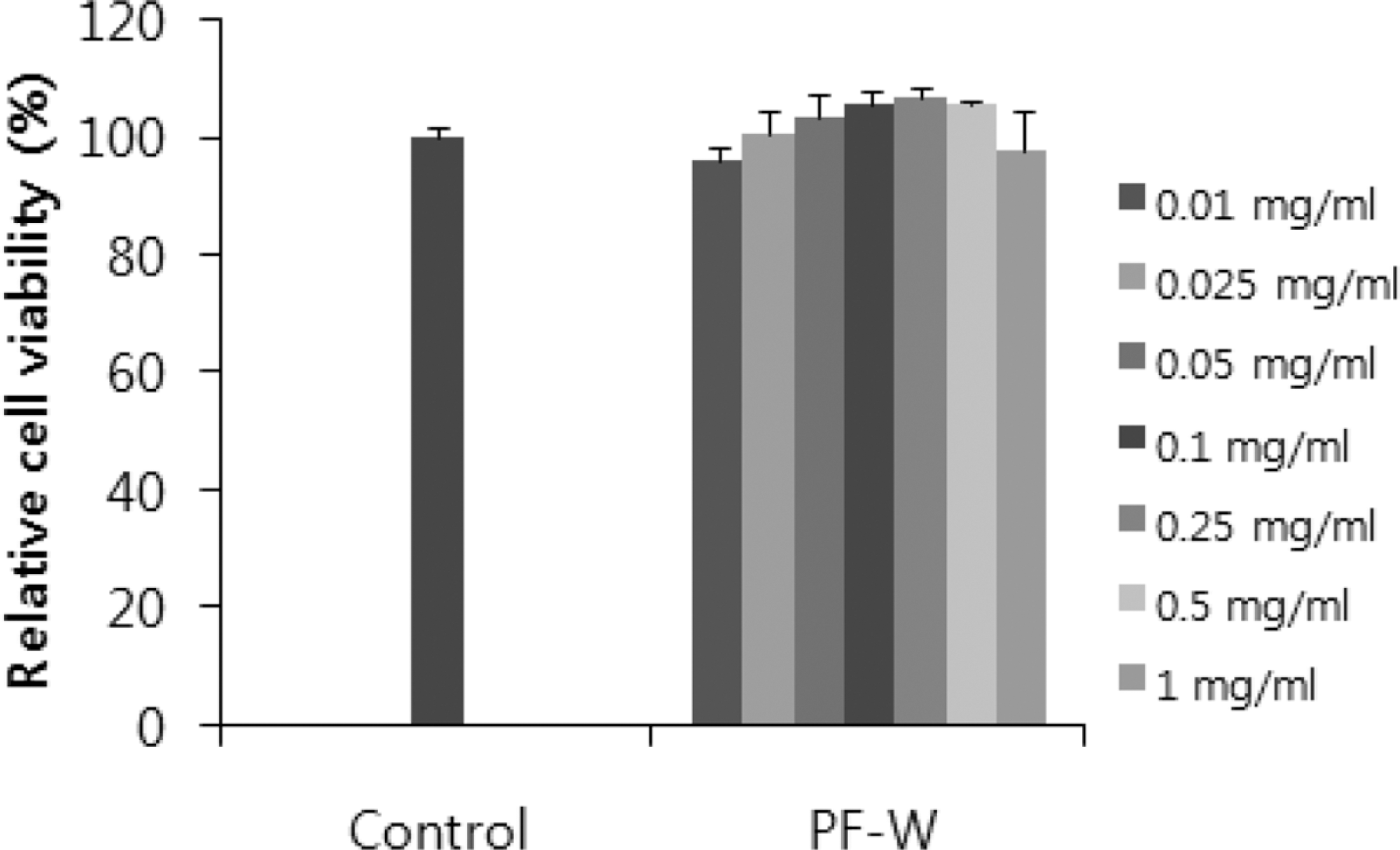 | Fig. 1.Effect of a water extract of the dried, immature fruit of Poncirus trifoliata (PF-W) on the viability of 3T3-L1 preadipocytes. 3T3-L1 cells were treated with PF-W (0.01, 0.025, 0.05, 0.1, 0.25, 0.5, or 1 mg/mL) for 24 h. Cell viability was determined using the Cell Counting Kit (CCK)-8. Data are expressed as Mean ± SD of triplicate experiments. |
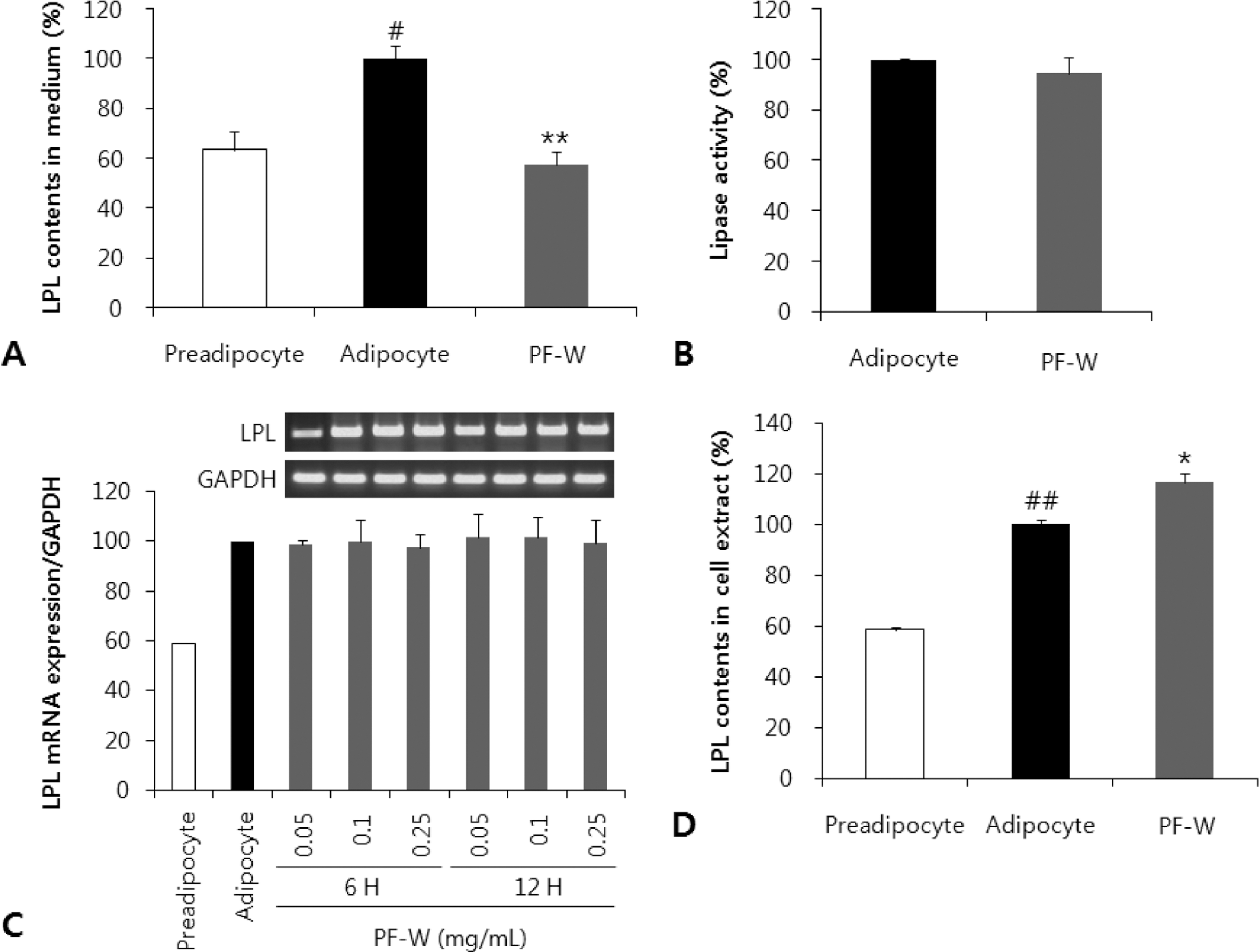 | Fig. 2.Effect of a water extract of the dried, immature fruit of Poncirus trifoliata (PF-W) on the lipoprotein lipase (LPL) expression and activity in 3T3-L1 adipocytes. A: LPL content of 3T3-L1 cell culture medium. B: Comparison of LPL activities in cell extract with and without 0.25 mg/mL PF-W. C: LPL mRNA expression as a function of PF-W concentration and time of exposure. D: LPL content in 3T3-L1 cell extract. Data are expressed as Mean ± SD of triplicate experiments. #: p < 0.05, ##: p < 0.01 as compared to the preadipocytes. ∗: p < 0.05, ∗∗: p < 0.01 compared to control adipocytes. |
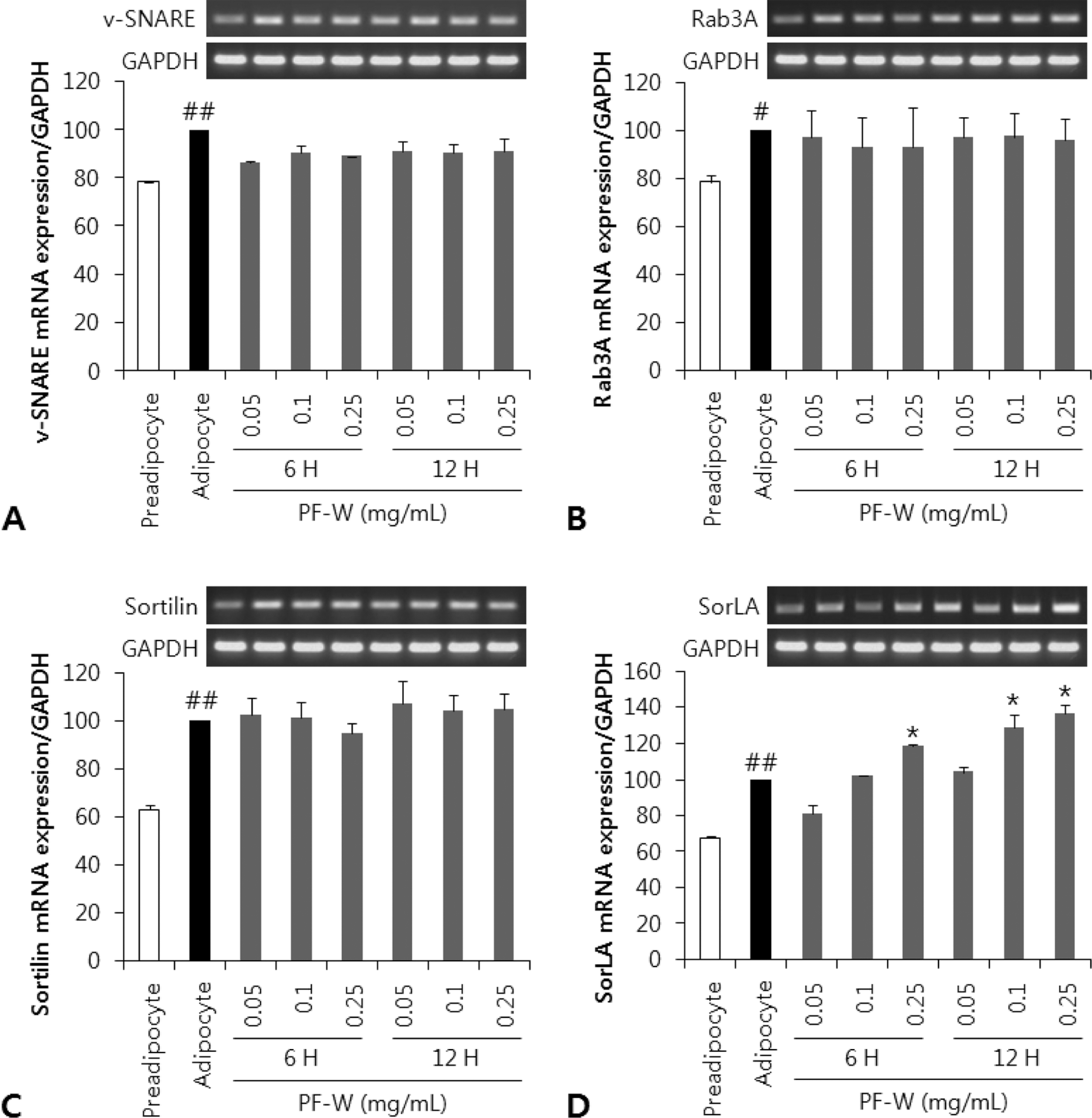 | Fig. 3.Effect of a water extract of the dried, immature fruit of Poncirus trifoliata (PF-W) on the mRNA expression levels of protein transport-related biomarkers in 3T3-L1 adipocytes. 3T3-L1 cells were treated with PF-W (0.25 mg/mL) for 6 and 12 h. A: v-SNARE mRNA expression. B: Rab3A mRNA expression. C: Sortilin (SorLA) mRNA expression. D: SorLA mRNA expression. Data are expressed as Mean ± SD of triplicate experiments. #: p < 0.05, ##: p < 0.01 compared to the preadipocyte. ∗: p < 0.05 compared to the adipocyte. |
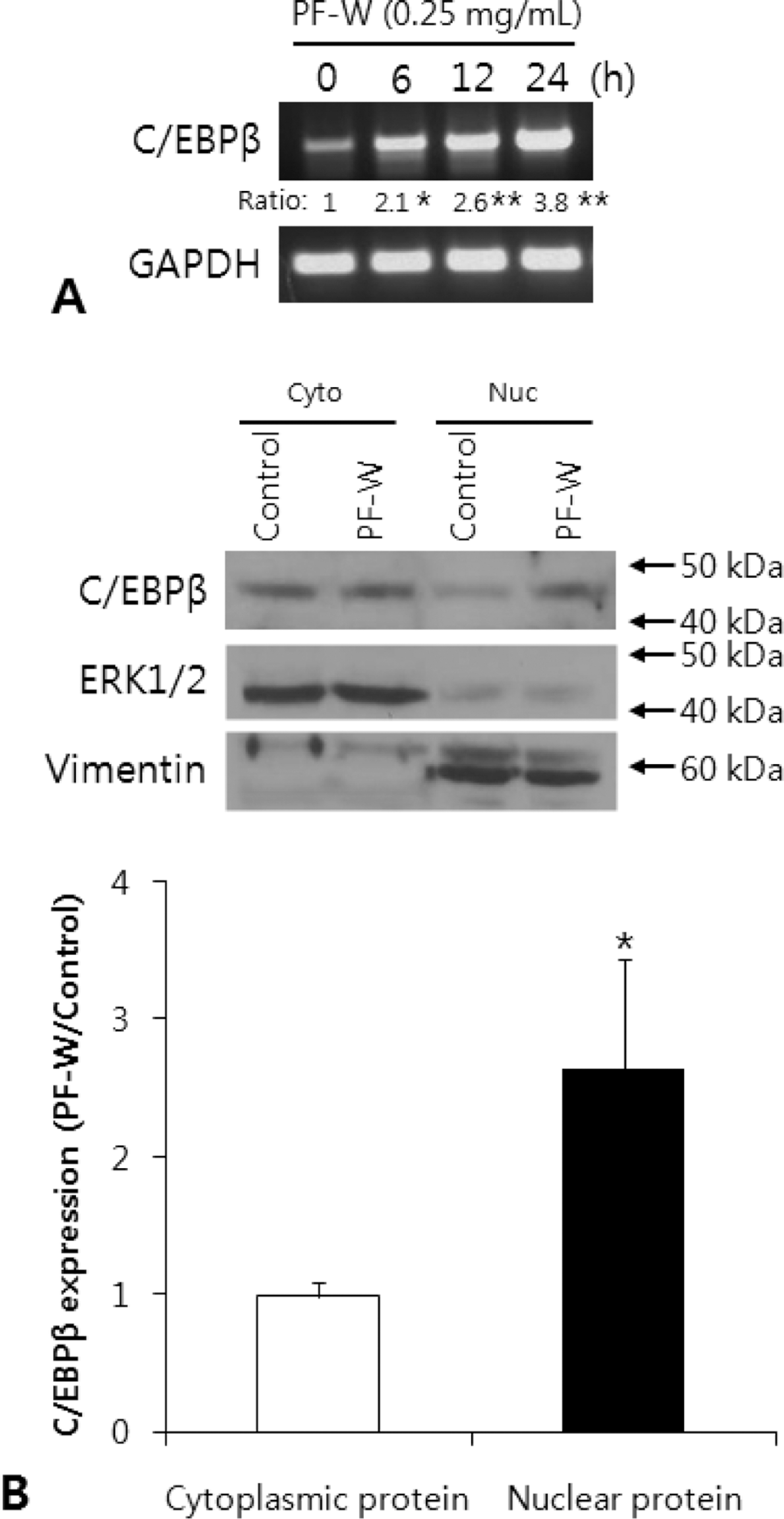 | Fig. 4.Effect of a water extract of the dried, immature fruit of Poncirus trifoliata (PF-W) on C/EBPβ expression in 3T3-L1 adipocytes. 3T3-L1 cells were treated with PF-W (0.25 mg/mL) for 12 h. A: C/ EBPβ mRNA expression by PF-W treatment. B: C/EBPβ protein expression in cytoplasmic and nuclear protein fraction by PF-W treatment. Data are expressed as Mean ± SD of triplicate experiments. ∗: p < 0.05 and ∗∗: p < 0.01. |
Table 1.
PCR primer sets and expected sizes of PCR products use in the experiment
Table 2.
PCR condition of each primer sets
Table 3.
The contents of total polyphenol and flavonoid of Poncirus trifoliata water extract (PF-W)
| Sample | Total polyphenol (mg/g, GAE1)) | Total flavonoid (mg/g, QE2)) |
|---|---|---|
| PF-W | 52.15 ± 4.02 | 6.56 ± 0.47 |
Table 4.
The half maximal inhibitory concentration (IC50) of Ponci rus trifoliata water extract (PFW)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download