Abstract
Purpose:
The aim of this study is to investigate anti-arthritis activity using natural eggshell membrane (NEM).
Methods:
NEM was administered at 52 mg/kg, 200 mg/kg, and 400 mg/kg to SD-Rat, where arthritis was induced by monosodium iodoacetate (MIA) at 3 mg. NO production in serum was measured using Griess reagent. Cytokines including IL-1β, and IL-6 were measured by Luminex and PGE2, MMP-2, MMP-9, TIMP-1, LTB4, and hs-CRP were measured by ELISA. The cartilage of patella volume was examined and 3-D high-resolution reconstructions of the cartilage of patella were obtained using a Micro-CT system.
Results:
Production of NO, IL-1β, IL-6, PGE2, MMP-2, MMP-9, TIMP-1, LTB4, and hs-CRP in serum was decreased, respectively, in comparison with control. The cartilage of patella volume increased significantly. In addition, the NEM group showed a decrease in the cartilage of patella, synovial membrane, and transformation of fibrous tissue.
REFERENCES
1.Korean College of Rheumatology. KCR textbook of rheumatology. Seoul: Koonja;2014.
2.The Society of Korean Medicine Rehabilitation. Oriental rehabilitation medicine, 2nd revision. Seoul: Koonja;2005.
3.Yoo MC. The latest trend in treating arthritis. J Rheumatol Health. 1995. 2(2):227–229.
4.Auw Yang KG., Saris DB., Dhert WJ., Verbout AJ. Osteoarthritis of the knee: current treatment options and future directions. Curr Orthop. 2004. 18(4):311–320.
5.Yang CH. Gastrointestinal disorders associated with non-steroidal anti-inflammatory drugs (NSAIDs). Dongguk J Med. 2003. 10(2):190–199.
6.Han JD., Shin JH., Hwang HC., Kim DY., Cho WH., Im GI. Interaction of cytokines in osteolysis. J Korean Orthop Res Soc. 2000. 3(2):125–132.
7.Lee JW., Do JH. Market trend of health functional food and the prospect of ginseng market. J Ginseng Res. 2005. 29(4):206–214.
8.Kim HK. Current status and prospect of nutraceuticals. Food Ind Nutr. 2004. 9(1):1–14.
9.Leach RM Jr., Rucker RB., Van Dyke GP. Egg shell membrane protein: a nonelastin desmosine/isodesmosine-containing protein. Arch Biochem Biophys. 1981. 207(2):353–359.
10.Okubo T., Akachi S., Hatta H. Structure of hen eggs and physiology of egg laying. Yamamoto T, Juneja LR, Hatta H, Kim M, editors. editors.Hen Eggs: Their Basic and Applied Science. Boca Raton (FL): CRC Press;1997. p. 1–12.

11.Jeon TW., Park KM. Functional properties of egg shell membrane hydrolysate as a food material. J Korean Soc Food Sci Anim Resour. 2002. 22(3):267–273.
12.Ruff KJ., DeVore DP. Reduction of pro-inflammatory cytokines in rats following 7-day oral supplementation with a proprietary eggshell membrane-derived product. Mod Res Inflamm. 2014. 3(1):19–25.

13.Ruff KJ., Winkler A., Jackson RW., DeVore DP., Ritz BW. Eggshell membrane in the treatment of pain and stiffness from osteoarthritis of the knee: a randomized, multicenter, double-blind, placebo-controlled clinical study. Clin Rheumatol. 2009. 28(8):907–914.

14.Jung YO. Pathogenesis of osteoarthritis. Diagn Treat. 2007. 27(4):395–399.
15.Kim CS., Park YK. The therapeutic effect of Achyranthis Radix on the collagen-induced arthritis in mice. Korean J Herbol. 2010. 25(4):129–135.
16.Wu W., Xu X., Dai Y., Xia L. Therapeutic effect of the saponin fraction from Clematis chinensis Osbeck roots on osteoarthritis induced by monosodium iodoacetate through protecting articular cartilage. Phytother Res. 2010. 24(4):538–546.

17.Wesche-Soldato DE., Swan RZ., Chung CS., Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007. 8(4):493–500.

18.Herrington C., Hall PA. Molecular and cellular themes in inflammation and immunology. J Pathol. 2008. 214(2):123–125.

19.Campo GM., Avenoso A., Campo S., D'Ascola A., Traina P., Samà D., Calatroni A. Glycosaminoglycans modulate inflammation and apoptosis in LPS-treated chondrocytes. J Cell Biochem. 2009. 106(1):83–92.

20.Clancy RM., Amin AR., Abramson SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998. 41(7):1141–1151.

21.Rice-Evance C., Miller N., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997. 2(4):152–159.
22.Chang ST., Wu JH., Wang SY., Kang PL., Yang NS., Shyur LF. Antioxidant activity of extracts from Acacia confusa bark and heartwood. J Agric Food Chem. 2001. 49(7):3420–3424.

23.Hardy MM., Seibert K., Manning PT., Currie MG., Woerner BM., Edwards D., Koki A., Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002. 46(7):1789–1803.

24.Okamoto H., Hoshi D., Kiire A., Yamanaka H., Kamatani N. Molecular targets of rheumatoid arthritis. Inflamm Allergy Drug Targets. 2008. 7(1):53–66.

25.Scheinecker C., Redlich K., Smolen JS. Cytokines as therapeutic targets: advances and limitations. Immunity. 2008. 28(4):440–444.

26.Ji JD. Cytokines in rheumatoid arthritis. Hanyang Med Rev. 2005. 25(2):43–52.
27.Hulejová H., Baresová V., Klézl Z., Polanská M., Adam M., Senolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007. 38(3):151–156.

28.LeGrand A., Fermor B., Fink C., Pisetsky DS., Weinberg JB., Vail TP., Guilak F. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001. 44(9):2078–2083.
29.Tak MJ., Tak MR., Kang KH., Ko WS., Yoon HJ. The Inhibitory effects of Yang Geouk San Hwa-Tang on LPS-stimulated inflammation in RAW264.7 macrophage cells. J Korean Orient Med Ophthalmol Otolaryngol Dermatol. 2010. 23(1):118–134.
30.Madson KL., Moore TL., Lawrence JM 3rd., Osborn TG. Cytokine levels in serum and synovial fluid of patients with juvenile rheumatoid arthritis. J Rheumatol. 1994. 21(12):2359–2363.
31.Yeo HJ., Lee JH., Lee HJ., Byun KS., Im HJ., Kim MJ. Microvascular findings in patients with rheumatoid arthritis: assessed, using fundus photography and fluorescein angiography. J Rheum Dis. 2013. 20(4):231–237.
32.Madhok R., Crilly A., Watson J., Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993. 52(3):232–234.

33.Reijman M., Hazes JM., Bierma-Zeinstra SM., Koes BW., Christgau S., Christiansen C., Uitterlinden AG., Pols HA. A new marker for osteoarthritis: cross-sectional and longitudinal approach. Arthritis Rheum. 2004. 50(8):2471–2478.

34.Garnero P., Peterfy C., Zaim S., Schoenharting M. Bone marrow abnormalities on magnetic resonance imaging are associated with type II collagen degradation in knee osteoarthritis: a three-month longitudinal study. Arthritis Rheum. 2005. 52(9):2822–2829.

35.Elsaid KA., Chichester CO. Review: collagen markers in early arthritic diseases. Clin Chim Acta. 2006. 365(1-2):68–77.

36.Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis. J Rheumatol. 1999. 26(3):717–719.
Fig. 1.
Effects of NEM on levels of NO in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗∗∗p < 0.001). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
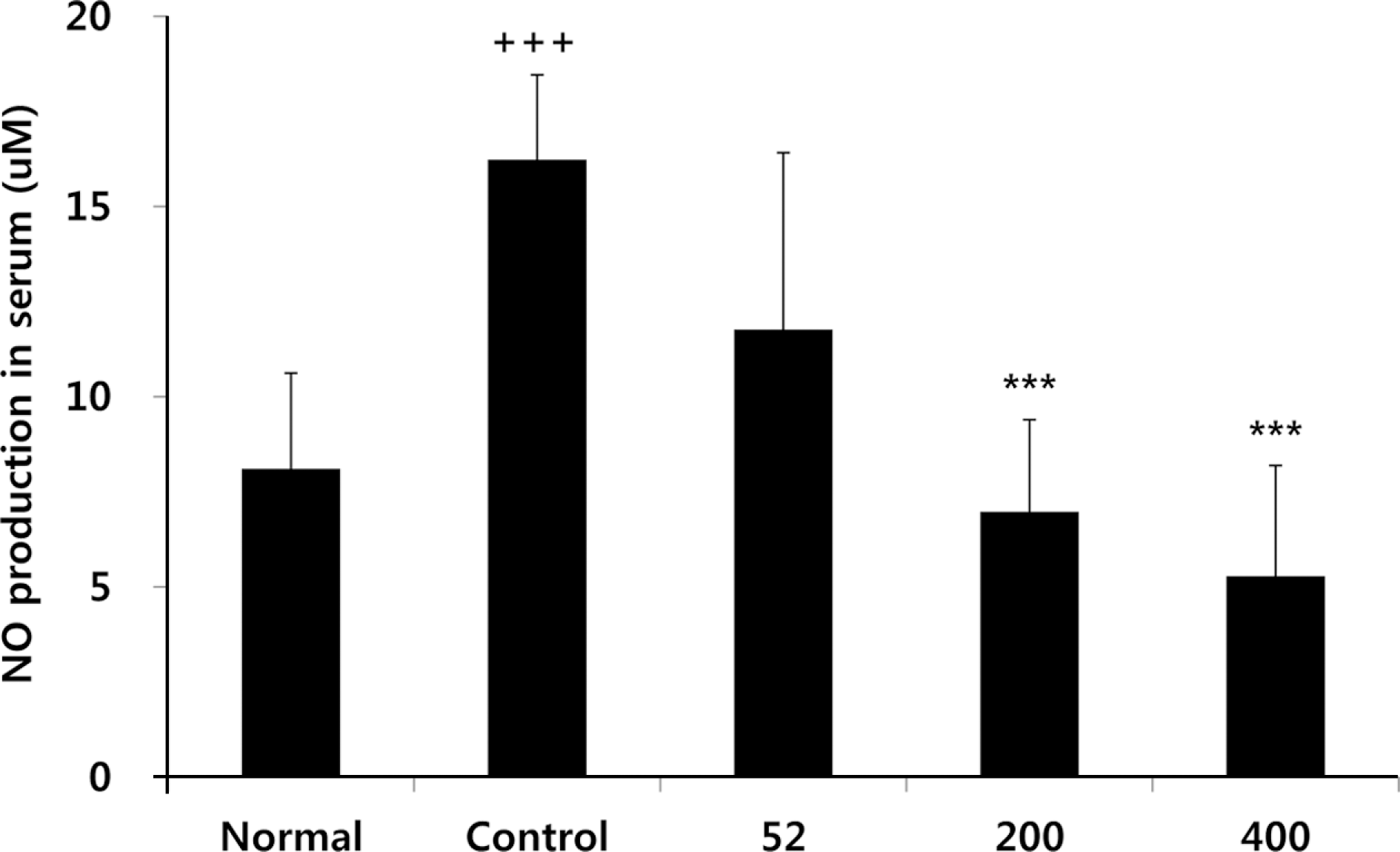
Fig. 2.
Effects of NEM on levels of PGE2 in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗p < 0.05, ∗∗p < 0.01). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/ kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
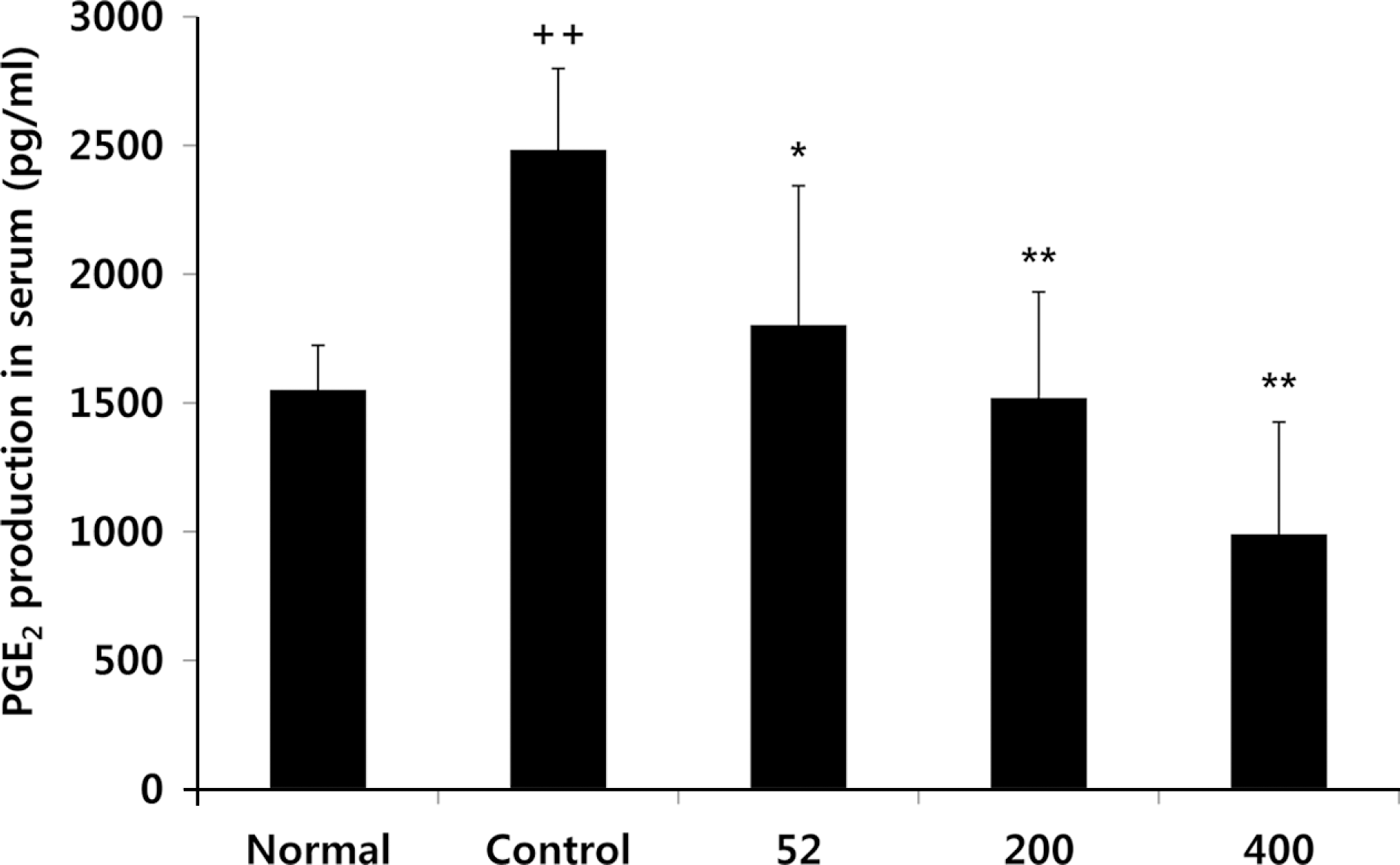
Fig. 3.
Effects of NEM on levels of IL-1ß, IL-6 in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA -induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
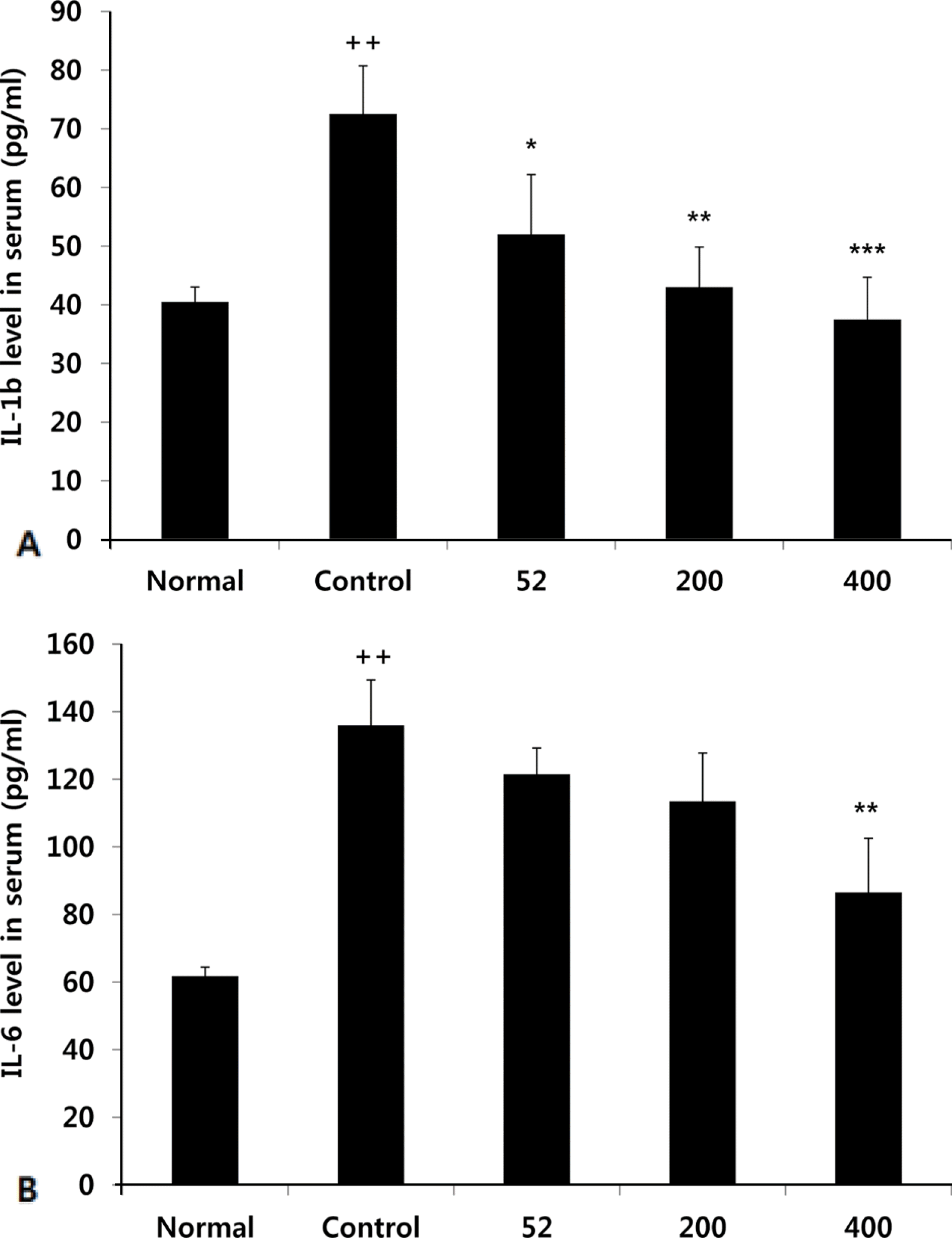
Fig. 4.
Effects of NEM on levels of hs-CRP in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗∗∗p < 0.001). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/ kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
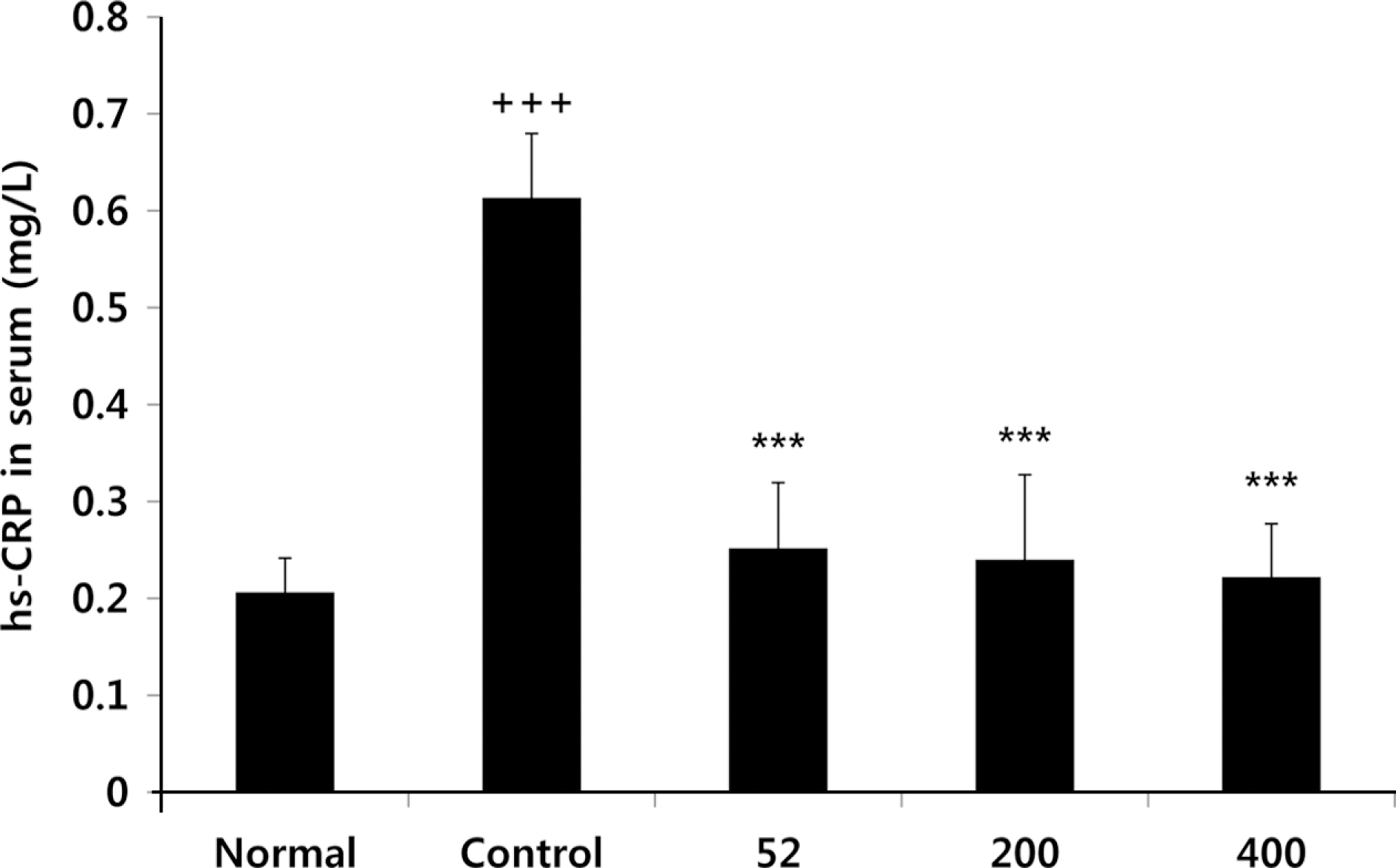
Fig. 5.
Effects of NEM on levels of MMP-2, MMP-9 in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗p < 0.05, ∗∗p < 0.01). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA (3 mg/mL)-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
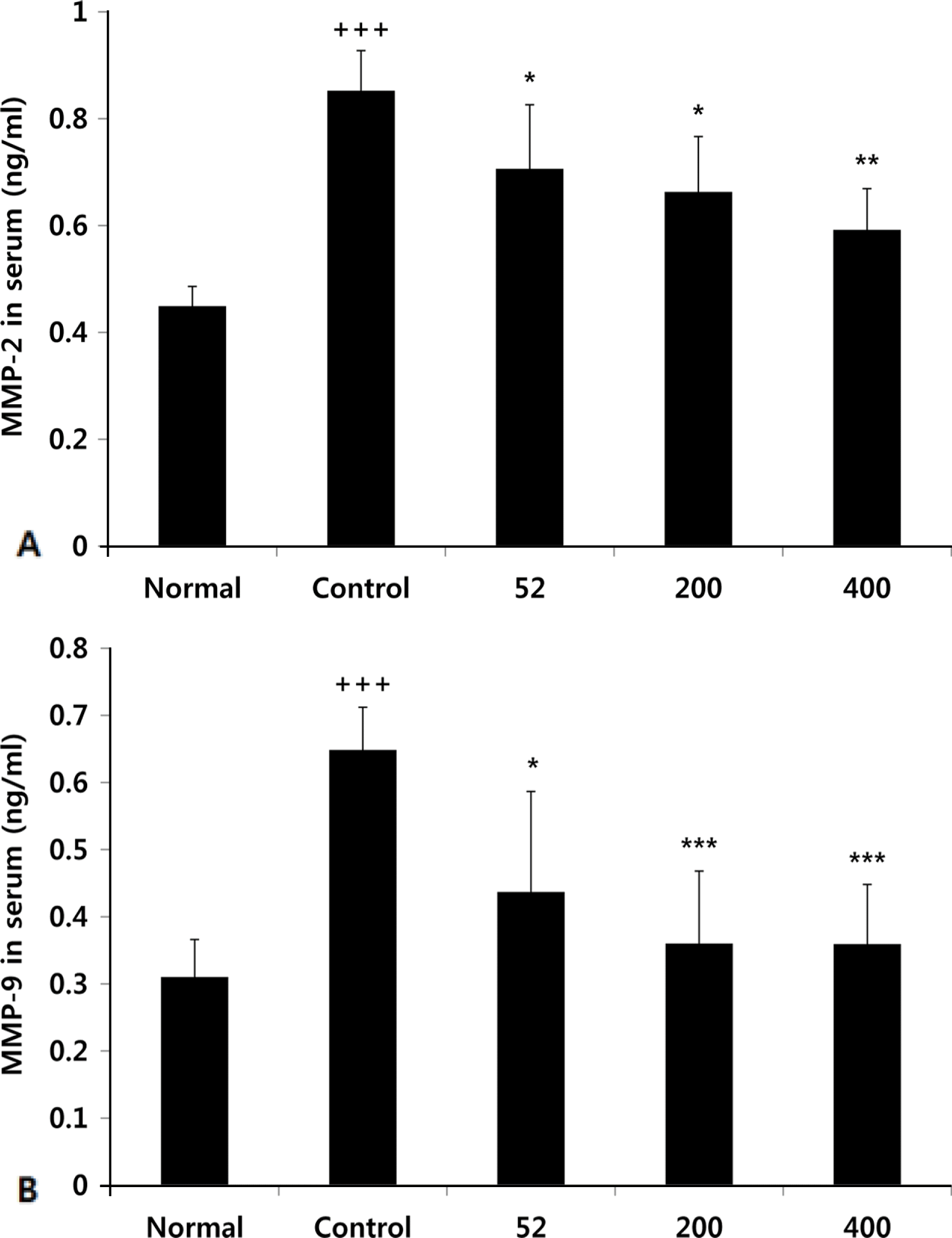
Fig. 6.
Effects of NEM on levels of LTB4 in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗∗p < 0.01, ∗∗∗p < 0.001). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
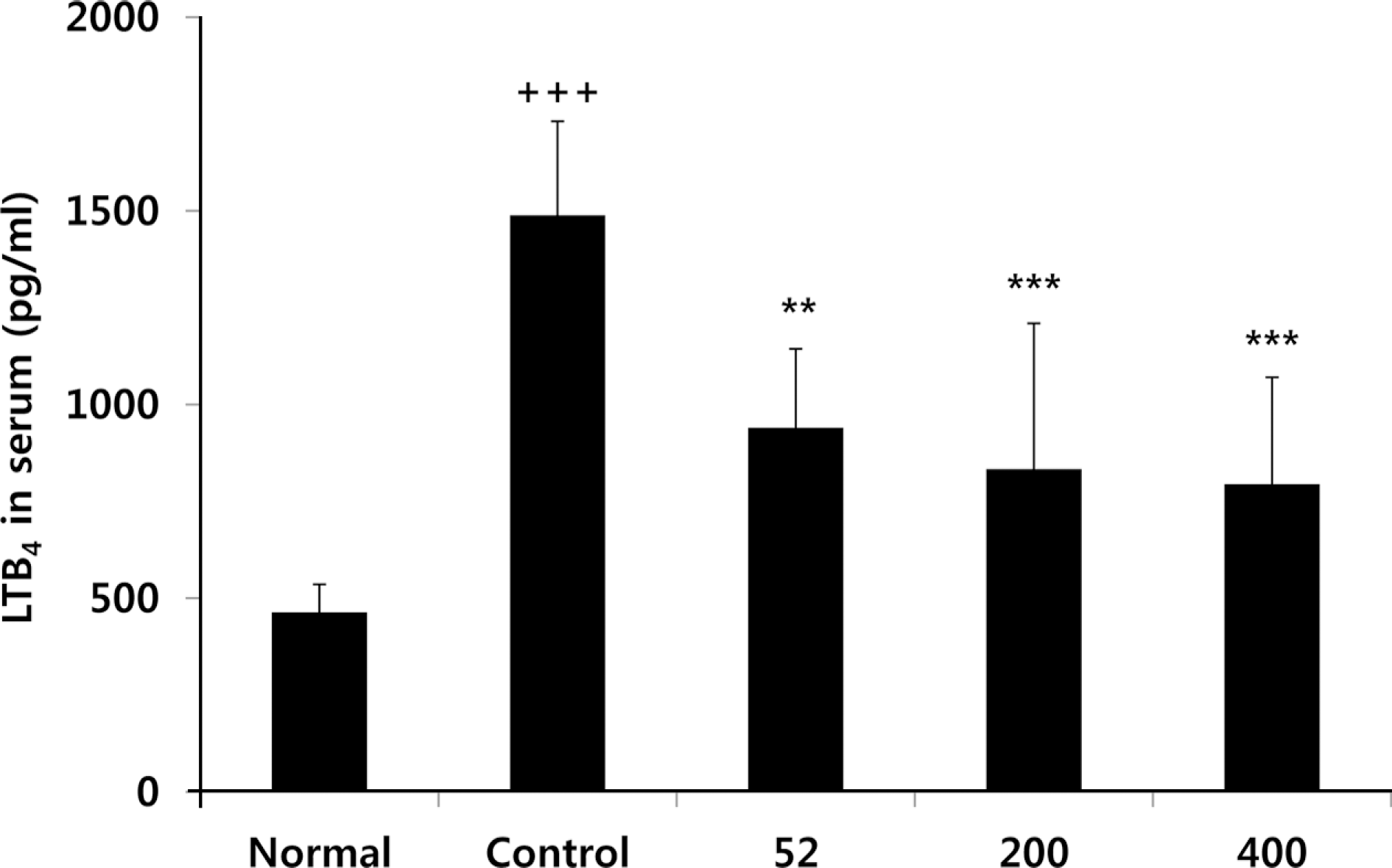
Fig. 7.
Effects of NEM on levels of COMP in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗p < 0.05). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
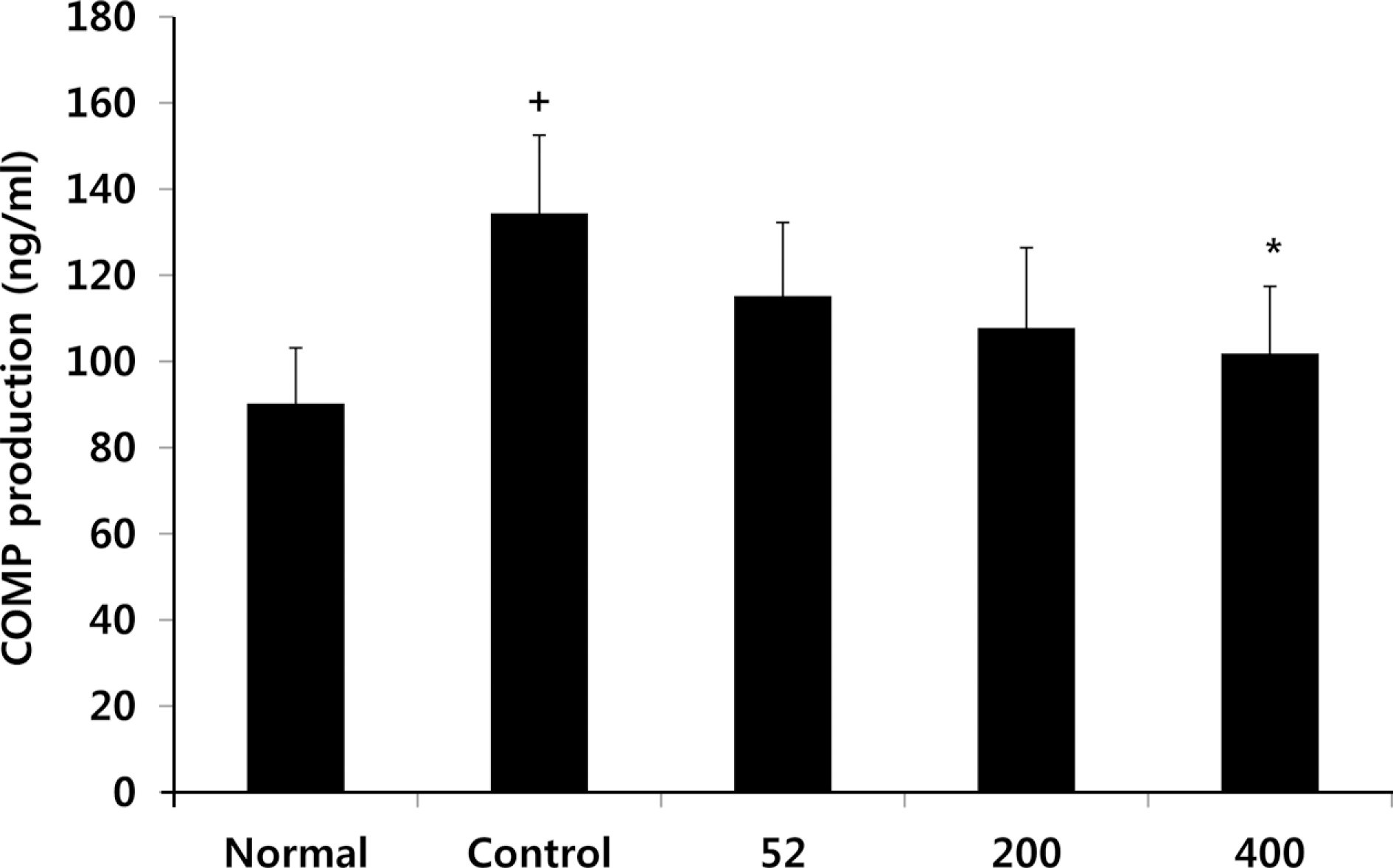
Fig. 8.
Effects of NEM on levels of CTX-II in the serum of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗∗p < 0.01). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
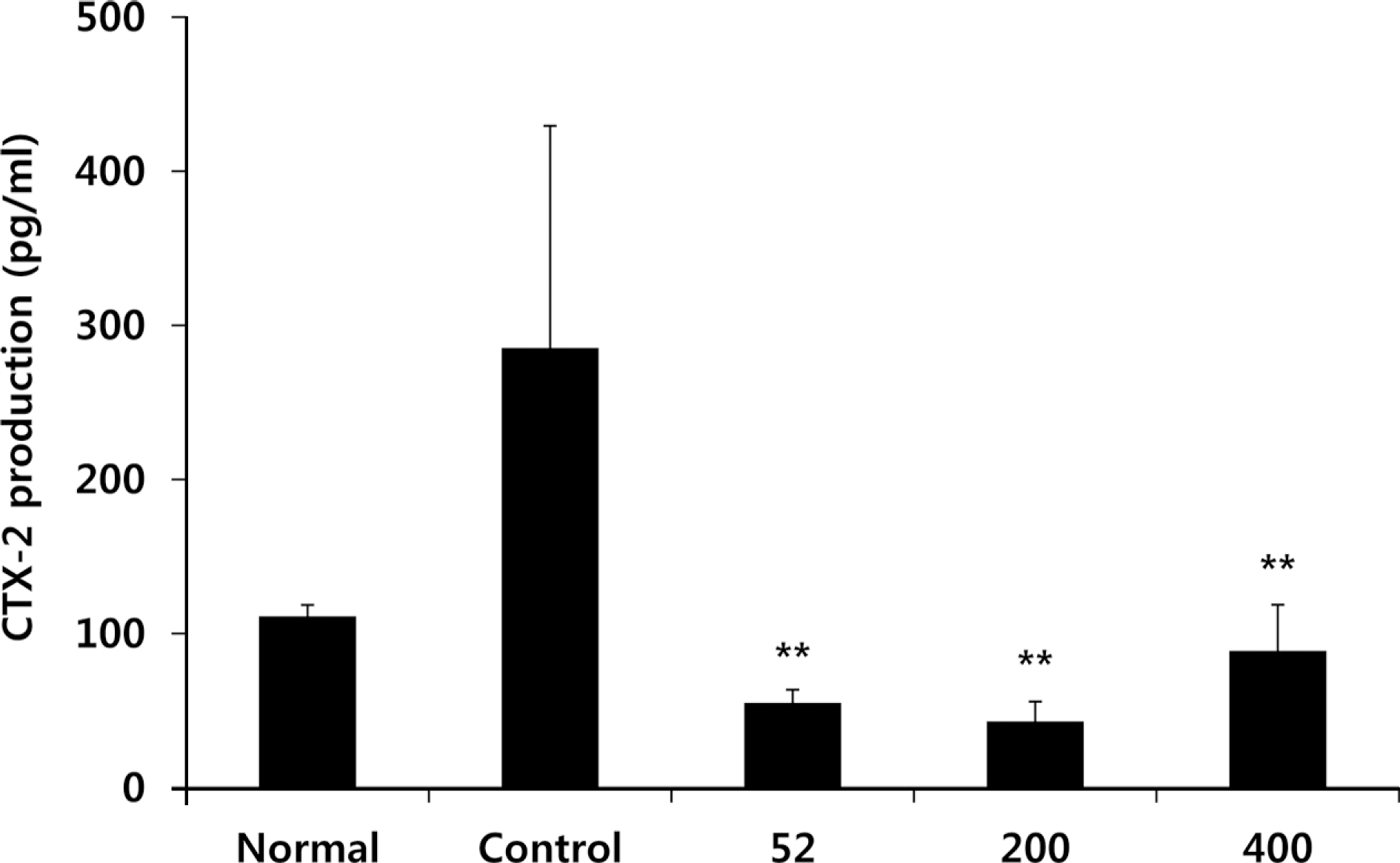
Fig. 9.
Effects of NEM on joint pathology (Hematoxylin & Eosin staining) and (Safranin-O) from joint tissue of MIA-induced osteoarthritis rats. Normal group shows the presence of slightly thickened synovium (arrow) Control group shows isolated areas with chronic inflammation (arrow). NEM treated group shows signs of tissue integrity with a thick layer of cartilage compared with control group (arrow). Normal, Normal SD-rat group; Control, MIA - induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.
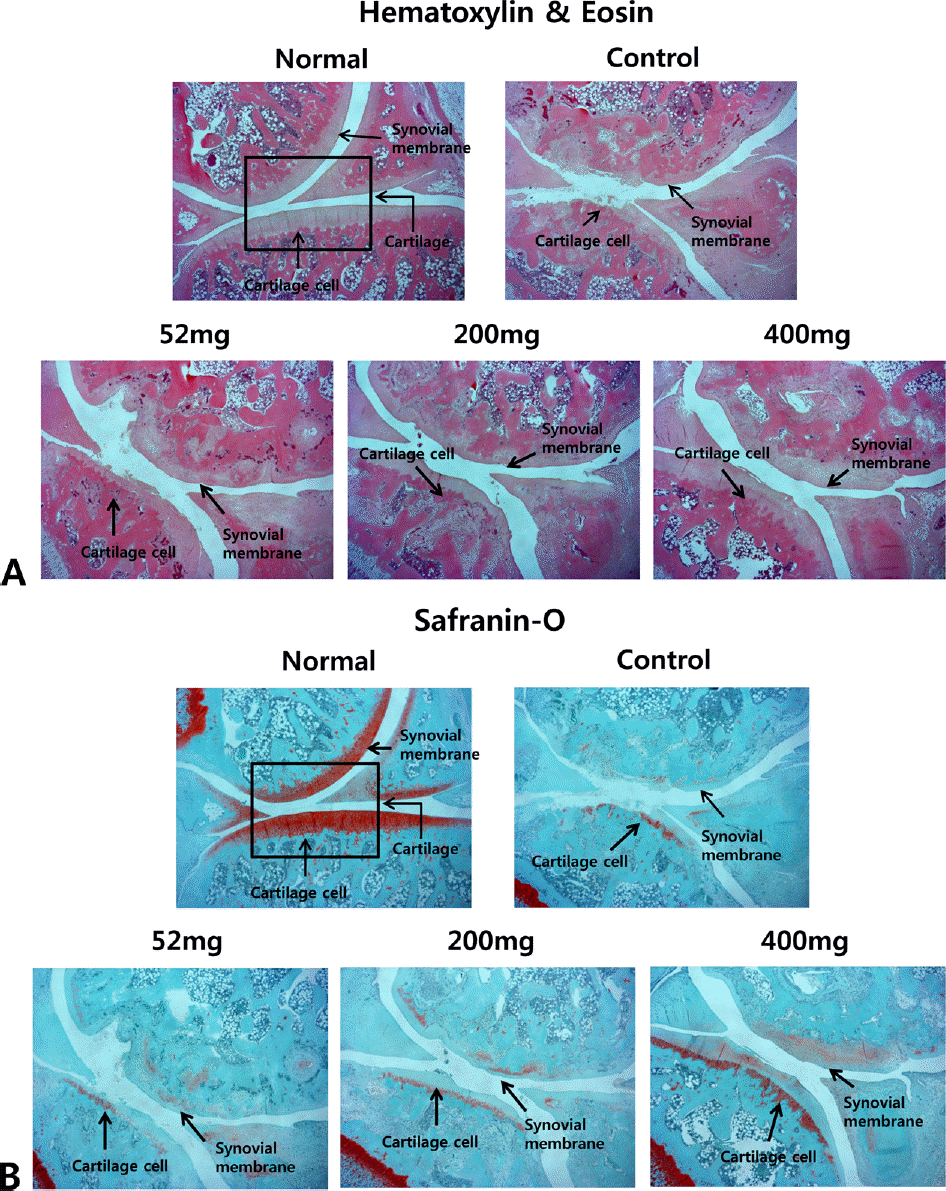
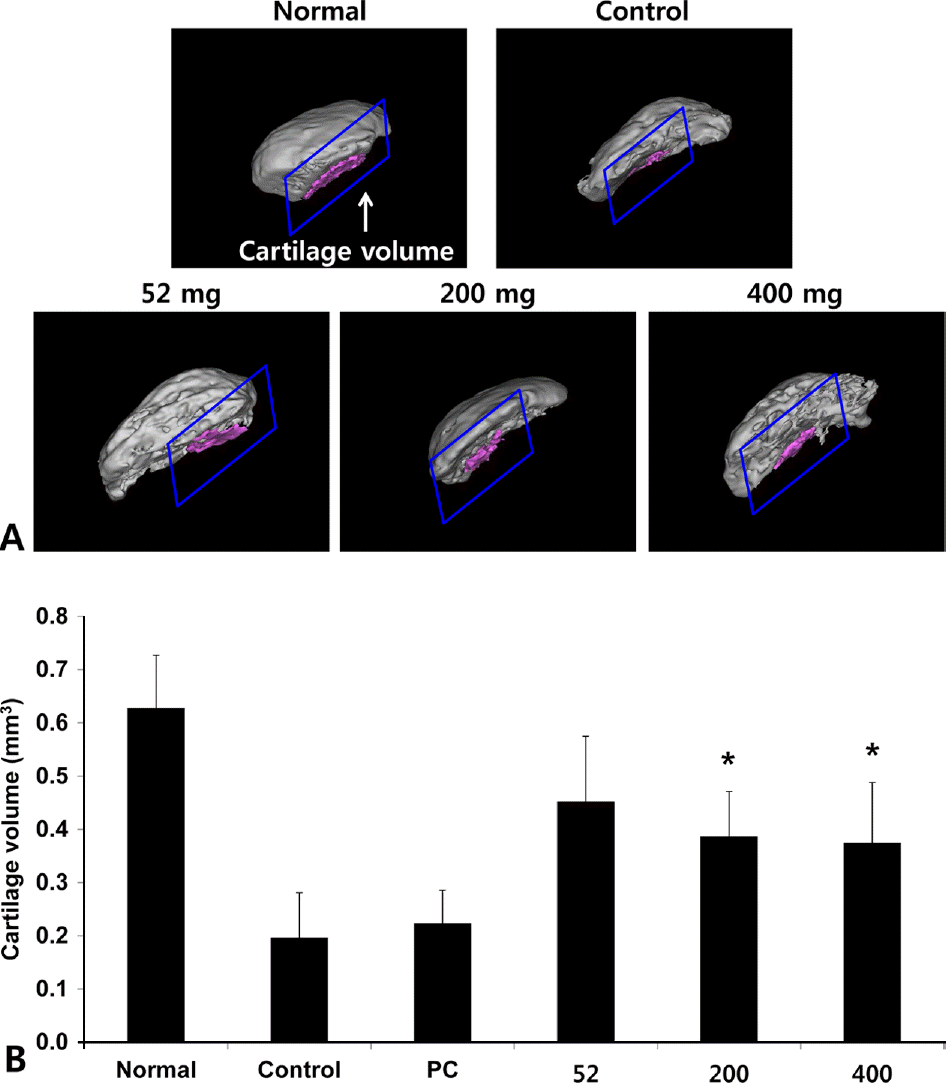
Fig. 10.
Effects of NEM on imaging of cartilage degeneration using micro CT-arthrography in joint tissue of MIA-induced osteoarthritis rat. The results were expressed as mean ± SD from 7 osteoarthritis rats. Statistically significant value compared with control group by unpaired student's t-test (∗p < 0.05). Normal, Normal SD-rat group; Control, MIA-induced osteoarthritis group; 52, MIA-induced osteoarthritis group + NEM 52 mg/kg; 200, MIA-induced osteoarthritis group + NEM 200 mg/kg; 400, MIA-induced osteoarthritis group + NEM 400 mg/kg.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download