Abstract
Purpose:
The purpose of this study was to examine the effect of black soybean (CJ-3) testa extracts on lipid profiles in streptozotocin (STZ)-induced diabetic rats.
Methods:
One control group and four STZ-induced diabetic groups with different doses of black soybean (CJ-3) testa extracts treatment [0 mg/kg (diabetic control, EX), 250 mg/kg (EX-250), 500 mg/kg (EX-500), 1,000 mg/kg (EX-1000)] were orally administered for 4 weeks.
Results:
All CJ-3 treatment groups had remarkably lower serum triglyceride (TG) levels than that of EX group (p < 0.05) whereas hepatic TG contents did not show any differences. Results from serum total cholesterol (TC) concentrations of EX-250 and EX-1000 groups were decreased compared to EX group (p < 0.05). Furthermore, protein levels of 3-hydroxy-3-methyl-glutaryl-Coenzyme A (HMG-CoA) reductase from the liver decreased in all treatment groups (p < 0.05). However, significant differences were not observed in serum glucose and insulin, and glucose transporter 4 (GLUT-4) protein expression in skeletal muscle tissue.
REFERENCES
1.Statistics Korea. Diabetes: ≥30 years, by sex. Daejeon: Statistics Korea;2013.
2.Jung SH. Health and medicine. Seoul: Shinil books;2007.
3.Park KS. Insulin resistance and insulin resistance syndrome. Hanyang Med Rev. 2009. 29(2):130–133.
4.Park HR., Cho JS. Effects of a natural medicinal multi-plant extract on blood glucose, insulin levels, and serum malondialdehyde concentrations in streptozotocin-induced diabetic rats. J East Asian Soc Diet Life. 2007. 17(2):205–212.
5.Hong JH., Park MR., Rhee SJ. Effects of YK-209 mulberry leaves on HMG-CoA reductase and lipid composition of liver on streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2002. 31(5):826–833.
6.Urano S., Hoshi-Hashizume M., Tochigi N., Matsuo M., Shiraki M., Ito H. Vitamin E and the susceptibility of erythrocytes and reconstituted liposomes to oxidative stress in aged diabetics. Lipids. 1991. 26(1):58–61.

7.Bae EH., Moon GS. A study on the antioxidative activities of Korean soybeans. J Korean Soc Food Sci Nutr. 1997. 26(2):203–208.
9.Cui CB., Choi HT., Lee HJ., Moon SY., Kim SH., Lee BG., Lee DS., Ham SS. Hypoglycemic effect of the functional food manufactured by fermented soybean as main materials in streptozotosin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2004. 33(7):1126–1132.
10.Byun JS., Han YS., Lee SS. The effects of yellow soybean, black soybean, and sword bean on lipid levels and oxidative stress in ovariectomized rats. Int J Vitam Nutr Res. 2010. 80(2):97–106.

11.United States Department of Health & Human Services; United States Department of Agriculture. Scientific report of the 2015 dietary guidelines advisory committee [Internet]. Washington, D.C.: United States Department of Health & Human Services; United States Department of Agriculture;2015. [cited 2015 Jan 28]. Available from:. http://health.gov/dietaryguidelines/2015-scientific-report/PDFs/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf.
12.Ranilla LG., Genovese MI., Lajolo FM. Polyphenols and antioxidant capacity of seed coat and cotyledon from Brazilian and Peruvian bean cultivars (Phaseolus vulgaris L.). J Agric Food Chem. 2007. 55(1):90–98.

13.Kim SY., Wi HR., Choi S., Ha TJ., Lee BW., Lee M. Inhibitory effect of anthocyanin-rich black soybean testa (Glycine max (L.) Merr.) on the inflammation-induced adipogenesis in a DIO mouse model. J Funct Foods. 2015. 14:623–633.

14.Kim YH., Kim DS., Woo SS., Kim HH., Lee YS., Kim HS., Ko KO., Lee SK. Antioxidant activity and cytotoxicity on human cancer cells of anthocyanin extracted from black soybean. Korean J Crop Sci. 2008. 53(4):407–412.
15.Kim NY., Lee YD., Cho SC., Shin YC., Lee HY. Enhancement of anti-inflammation effect by fermentation process in Aronia mela-nocarpa (Michx.) Elliott extract. Korean J Med Crop Sci. 2014. 22(6):475–482.

16.Choung MG., Lim JD. Antioxidant, anticancer and immune activation of anthocyanin fraction from Rubus coreanus Miquel fruits (Bokbunja). Korean J Med Crop Sci. 2012. 20(4):259–269.

17.Kim HB. Quantification of cyanidin-3-glucoside (C3G) in mulberry fruits and grapes. Korean J Seric Sci. 2003. 45(1):1–5.
18.Lee JH., Kang NS., Shin SO., Shin SH., Lim SG., Suh DY., Baek IY., Park KY., Ha TJ. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009. 112(1):226–231.

19.Ha TJ., Lee MH., Park CH., Pae SB., Shim KB., Ko JM., Shin SO., Baek IY., Park KY. Identification and characterization of anthocyanins in yard-long beans (Vigna unguiculata ssp. sesquipedalis L.) by high-performance liquid chromatography with diode array detection and electrospray ionization/mass spectrometry (HPLC-DAD-ESI/MS) analysis. J Agric Food Chem. 2010. 58(4):2571–2576.

20.Nader GA., Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol (1985). 2001. 90(5):1936–1942.

21.Folch J., Lees M., Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226(1):497–509.

22.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990. 9(6):515–540.

23.Wasowicz W., Nève J., Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993. 39(12):2522–2526.

24.Lee HJ., Hwang EH. Effects of Artemisia capillaris Thunberg on the plasma and liver lipid metabolism in rats. J Nutr Health. 2002. 35(4):421–430.
25.Nizamutdinova IT., Jin YC., Chung JI., Shin SC., Lee SJ., Seo HG., Lee JH., Chang KC., Kim HJ. The anti-diabetic effect of anthocyanins in streptozotocin-induced diabetic rats through glucose transporter 4 regulation and prevention of insulin resistance and pancreatic apoptosis. Mol Nutr Food Res. 2009. 53(11):1419–1429.

26.Bucci D., Rodriguez-Gil JE., Vallorani C., Spinaci M., Galeati G., Tamanini C. GLUTs and mammalian sperm metabolism. J Androl. 2011. 32(4):348–355.

27.Morgan BJ., Chai SY., Albiston AL. GLUT4 associated proteins as therapeutic targets for diabetes. Recent Pat Endocr Metab Immune Drug Discov. 2011. 5(1):25–32.
28.Shin JH., Lee SJ., Seo JK., Lee HJ., Ju JC., Sung NJ. Effect of a combined extract of Orostachys japonicus with medicinal plants on the lipid composition of the liver and kidney from streptozotocin-induced diabetic rats. J Korean Soc Food Sci Nutr. 2012. 41(4):510–518.

29.Cho HK. Diabetes mellitus and disorder of lipid metabolism. J Korean Soc Endocrinol. 2006. 21(2):101–105.

30.Liao Z., Chen X., Wu M. Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic rats. Arch Pharm Res. 2010. 33(3):353–362.

31.Kwon SH., Ahn IS., Kim SO., Kong CS., Chung HY., Do MS., Park KY. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J Med Food. 2007. 10(3):552–556.

32.Ko MK., Kwon TW., Song YS. Effects of yellow and black soybeans on plasma and hepatic lipid composition and fecal lipid excretion in rats. J Korean Soc Food Sci Nutr. 1998. 27(1):126–131.
33.Lee JW., Lee SM., Gwak KS., Lee JY., Choi IG. Screening of edible mushrooms for the production of lovastatin and its HMG-CoA Reductase inhibitory activity. Korean J Microbiol. 2006. 42(2):83–88.
34.Jung JH., Kim HS. The inhibitory effect of black soybean on hepatic cholesterol accumulation in high cholesterol and high fat diet-induced non-alcoholic fatty liver disease. Food Chem Toxicol. 2013. 60:404–412.

35.Yu RQ., Wu XY., Zhou X., Zhu J., Ma LY. Cyanidin-3-glucoside attenuates body weight gain, serum lipid concentrations and insulin resistance in high-fat diet-induced obese rats. Zhongguo Dang Dai Er Ke Za Zhi. 2014. 16(5):534–538.
36.Shin SH., Park DS., Jang MJ., Jeon JH., O NG., Song JY., Lee JS., Kim BY., Joo SS., Hwang SY., Kim YB. Protective effect of anthocyanins against the hepatotoxicity of carbon tetrachloride in rats. Lab Anim Res. 2008. 24(3):355–360.
Fig. 1.
HPLC chromatogram and chemical structure of C3G and D3G. Peak 1; D3G (4.22 mg/g), peak 2; C3G (10.23 mg/g). HPLC condition [column; Gemini (C18, 3 µm, 4.6 mm × 100 mm), detector; DAD (530 nm), flow rate; 0.8 ml/min, solvent A; acetonitrille, solvent B; distilled water, solvent C; 0.5 M oxalic acid, gradient condition with 0~5 min; 10% A, 85% B, 5% C, 20 min; 25% A, 70% B, 5% C].
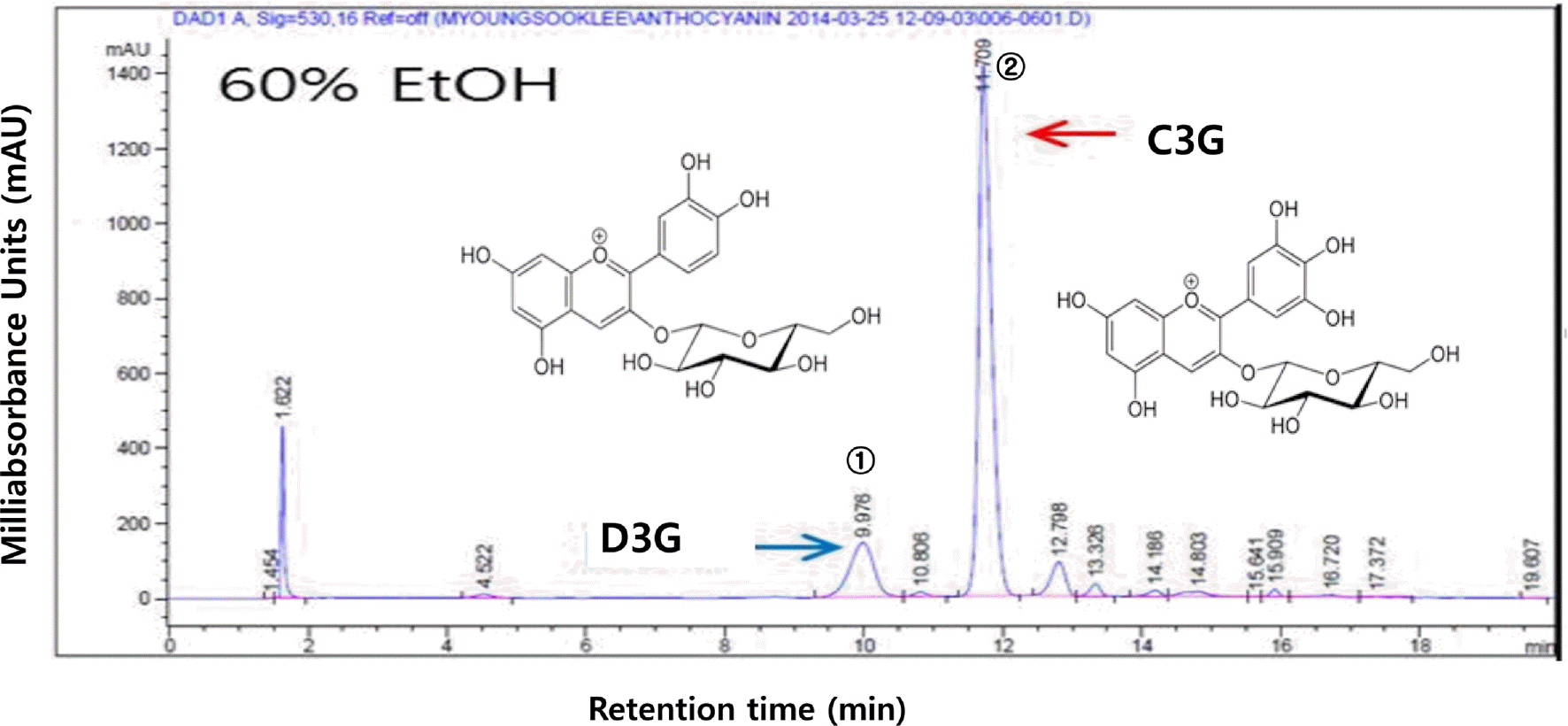
Fig. 2.
Organ weight of normal and diabetic rats fed black soybean testa extracts. (A) Liver weight (g/100 g body weight) and (B) Muscle weight (g/100 g body weight). Mean values ± SD with different letters represent significant difference at p < 0.05. CON; normal control, EX; STZ-induced diabetes (diabetic control), EX-250; black soybean testa extracts 250 mg/kg oral treatment, EX-500; black soybean testa extracts 500 mg/kg oral treatment, EX-1000; black soybean testa extracts 1,000 mg/kg oral treatment after diabetes induction by STZ treatment.
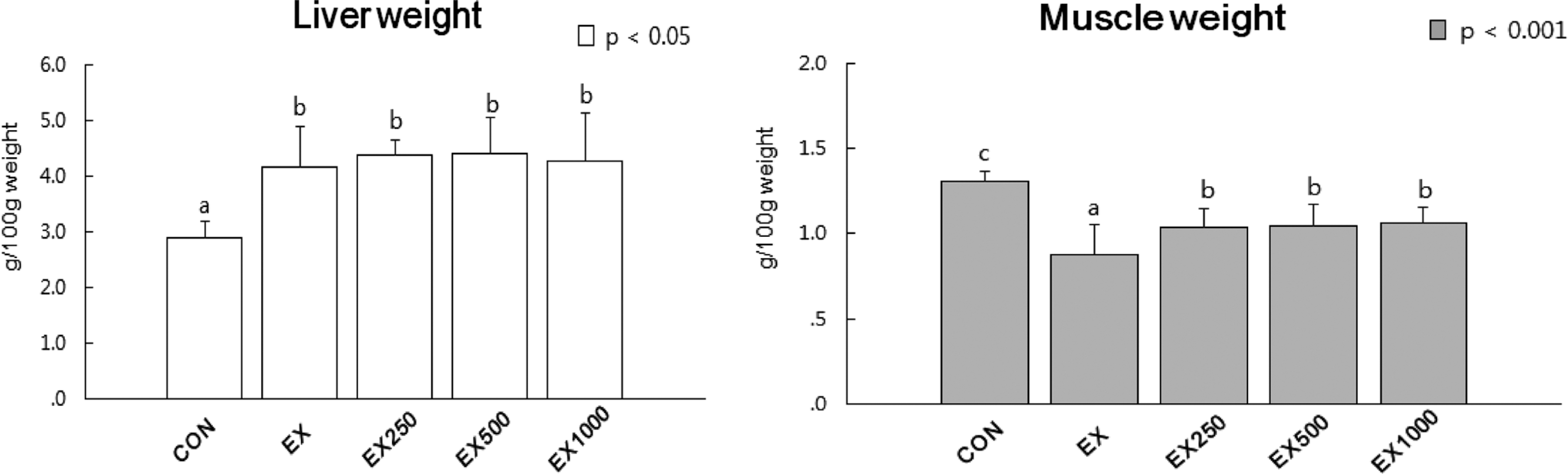
Fig. 3.
Effects of black soybean testa extracts on glucose metabolism. (A) Glucose levels in serum, (B) Insulin levels in serum (C) Glycogen level in liver (D) Glycogen level in muscle and (E) GLUT-4 protein expression levels in skeletal muscle tissue . Glycogen contents in liver and skeletal muscle. Mean values ± SD with different letters represent significant difference at p < 0.05. CON; normal control, EX; STZ-induced diabetes (diabetic control), EX-250; black soybean testa extracts 250 mg/kg oral treatment, EX-500; black soybean testa extracts 500 mg/kg oral treatment, EX-1000; black soybean testa extracts 1,000 mg/kg oral treatment after diabetes induction by STZ treatment.
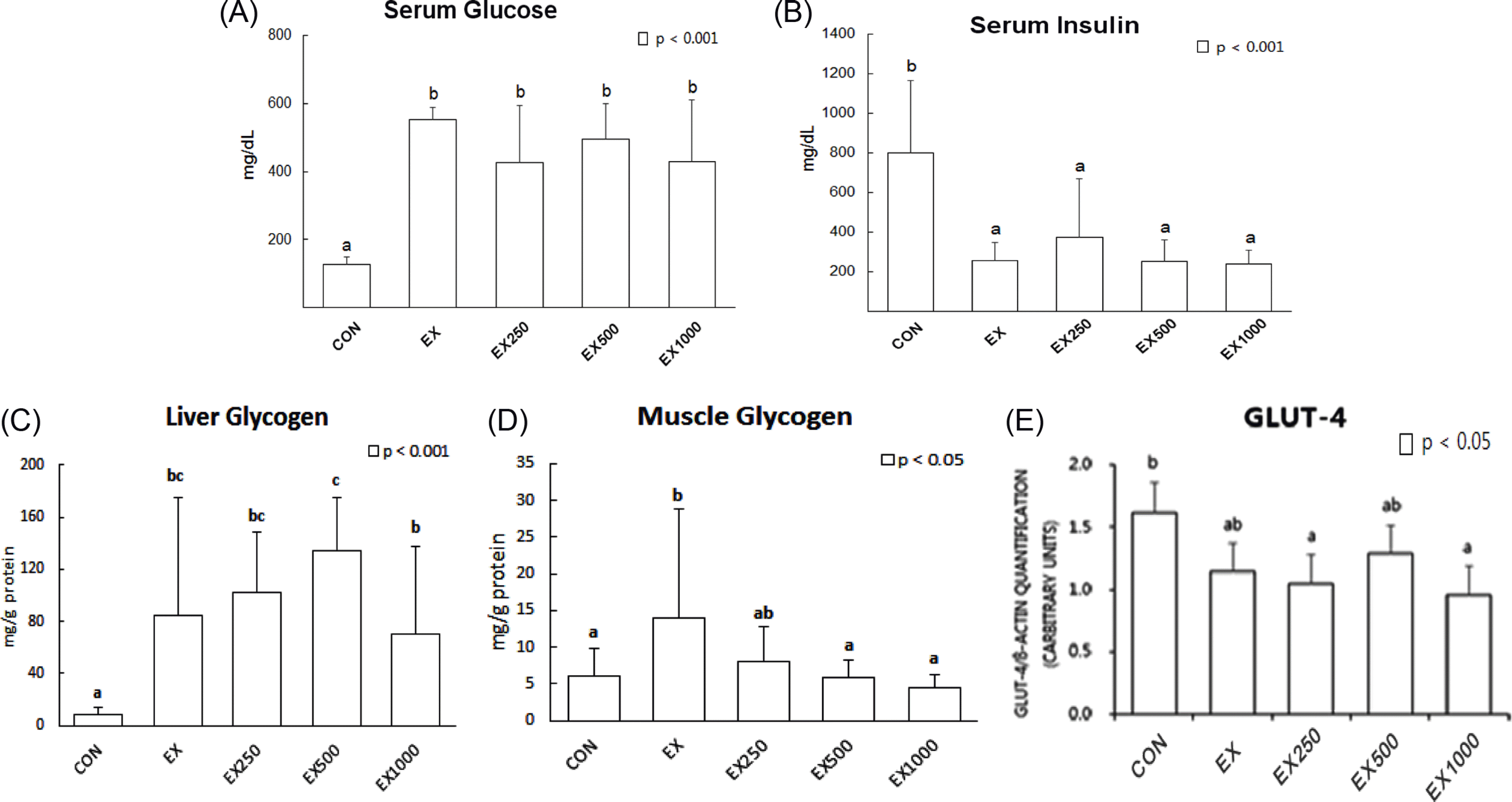
Fig. 4.
Effects of black soybean testa extracts on lipid profile and MDA. (A) TG levels in serum, (B) TG levels in liver, (C) TG levels in liver and (D) HMG-CoA reductase protein expression levels in liver and (E) MDA levels of serum Mean values ± SD with different letters represent significant difference at p < 0.05. CON; normal control, EX; STZ-induced diabetes (diabetic control), EX-250; black soybean testa extracts 250 mg/kg oral treatment, EX-500; black soybean testa extracts 500 mg/kg oral treatment, EX-1000; black soybean testa extracts 1,000 mg/kg oral treatment after diabetes induction by STZ treatment.
Fig. 5.
Histological findings from livers of each groups. The micrographs depict H&E of paraffin-embedded liver slides. A; normal control (CON), B; STZ-induced diabetes (EX, diabetic control), C; black soybean testa extracts 250 mg/kg oral treatment (EX-250), D; black soybean testa extracts 500 mg/kg oral treatment (EX-500) and E; black soybean testa extracts 1,000 mg/kg oral treatment (EX-1000). White arrow describes tissue necrosis around central hepatic veis. Black arrow describes the lipid globules occurred by STZ treatment.
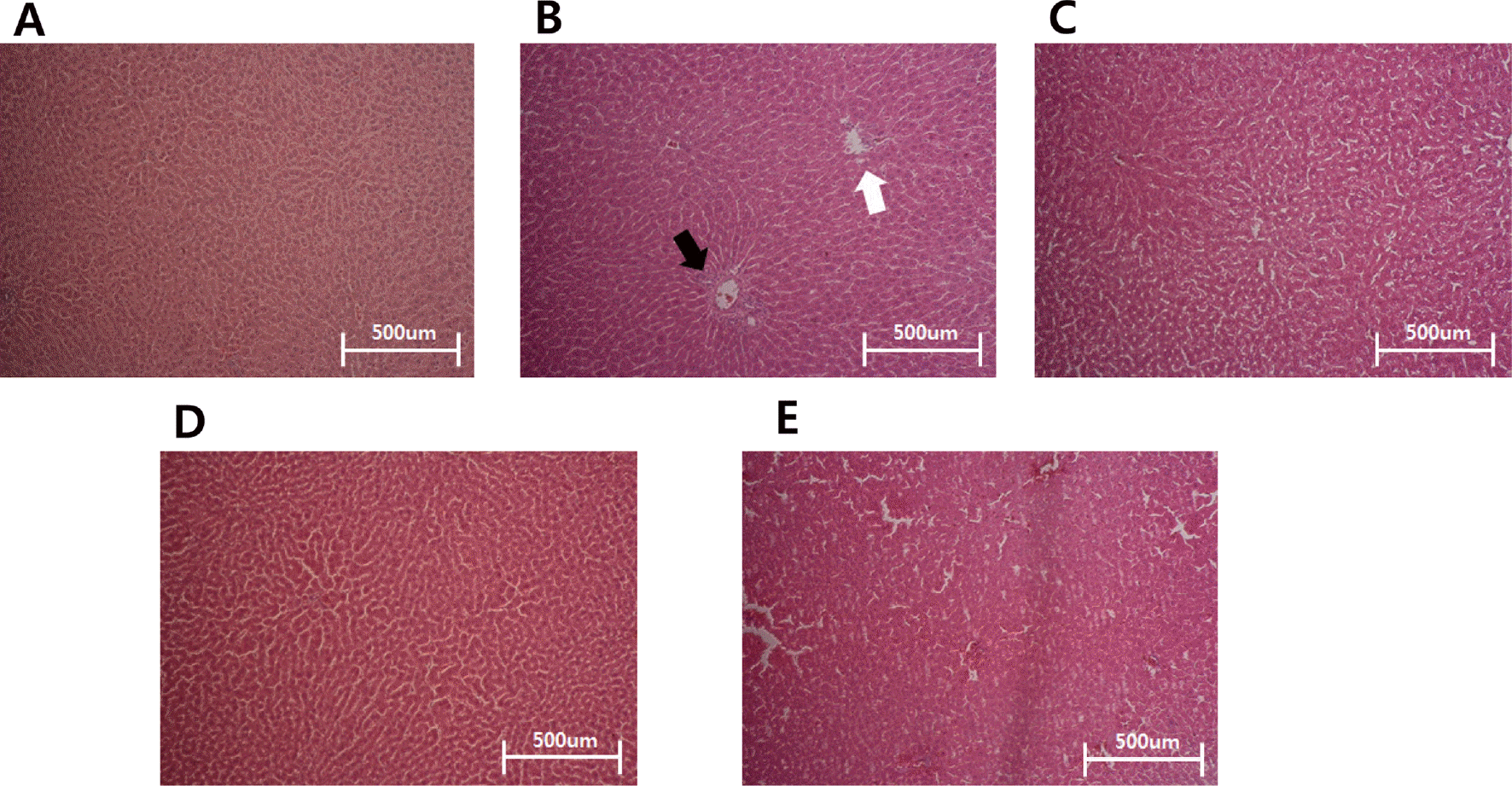
Table 1.
Experimental design in this study
| Groups1) | Oral treatment concentrations (mg/kg) | Type I diabetes induction | The number of individual (n) |
|---|---|---|---|
| CON | NaCl | - | 9 |
| EX | NaCl | STZ2) | 7 |
| EX-250 | 250 | STZ | 8 |
| EX-500 | 500 | STZ | 8 |
| EX-1000 | 1,000 | STZ | 8 |
Table 2.
Body weights, diet intake and feed efficiency ratio of normal and diabetic rats fed black soybean testa extracts
| Variables1) | Week 1 | Week 2 | Week 3 | Week 4 | p-value (period)∗ |
|---|---|---|---|---|---|
| Body weight (kg) | |||||
| CON (n = 9) | 205.4 ± 0.3ax‡ | 260.6 ± 14.7bx | 306.6 ± 0.7cx | 331.8 ± 1.5dx | p < 0.001 |
| EX (n = 7) | 135.9 ± 23.5y | 137.9 ± 34.2y | 146.8 ± 3.4y | 143.1 ± 6.2y | NS |
| EX-250 (n = 8) | 141.5 ± 17.4y | 136.2 ± 26.7y | 141.6 ± 2.0y | 141.8 ± 4.6y | NS |
| EX-500 (n = 8) | 141.3 ± 11.7y | 138.9 ± 28.2y | 142.2 ± 3.2y | 141.8 ± 6.6y | NS |
| EX-1000 (n = 8) | 147.1 ± 10.9y | 142.4 ± 22.3y | 144.9 ± 4.6y | 157.0 ± 6.7y | NS |
| p-value∗∗ | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Diet intakes (g) | |||||
| CON (n = 9) | 22.1 ± 0.7 | 23.1 ± 1.1 | 22.5 ± 1.5 | 22.7 ± 1.6 | NS |
| EX (n = 7) | 22.4 ± 4.2 | 27.0 ± 8.1 | 28.4 ± 6.9 | 28.0 ± 8.1 | NS |
| EX-250 (n = 8) | 23.7 ± 2.1 | 24.2 ± 4.6 | 25.2 ± 4.8 | 27.4 ± 7.5 | NS |
| EX-500 (n = 8) | 23.6 ± 3.4 | 26.6 ± 6.3 | 26.0 ± 5.3 | 26.8 ± 7.2 | NS |
| EX-1000 (n = 8) | 23.6 ± 4.6 | 27.6 ± 6.3 | 27.3 ± 6.7 | 27.7 ± 6.5 | NS |
| p-value∗∗ | NS | NS | NS | NS | |
| Feed efficiency ratio (%) | |||||
| CON (n = 9) | 2.65 ± 0.2dx | 1.93 ± 0.2cx | 1.32 ± 0.3bx | 0.89 ± 0.7a | p < 0.001 |
| EX (n = 7) | -0.03 ± 0.6y | 0.06 ± 0.3y | 0.06 ± 0.2y | -0.03 ± 0.2 | NS |
| EX-250 (n = 8) | -0.36 ± 0.7y | 0.07 ± 0.2y | -0.15 ± 1.4y | 0.22 ± 0.2 | NS |
| EX-500 (n = 8) | -0.21 ± 0.6y | -0.09 ± 0.2y | 0.32 ± 0.3y | 0.92 ± 1.9 | NS |
| EX-1000 (n = 8) | -0.50 ± 0.0.5ay | -0.06 ± 0.2by | 0.17 ± 0.2by | 0.10 ± 0.1b | p < 0.001 |
| p-value∗∗ | p < 0.001 | p < 0.001 | p < 0.001 | NS | |
1) Variables (five groups) - CON; contol, EX; STZ-induced diabetes, EX-250, EX-500 and EX-1000 describe oral treatments of black soybean testa extracts with 250 mg/kg, 500 mg/kg and 1,000 mg/kg for 4 weeks after STZ induction.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download