Abstract
Purpose:
The effects of water extract and distillate from the mixture of black goat meat and medicinal herb on MG-63 osteoblast proliferation and mouse bone marrow derived osteoclast formation were investigated.
Methods:
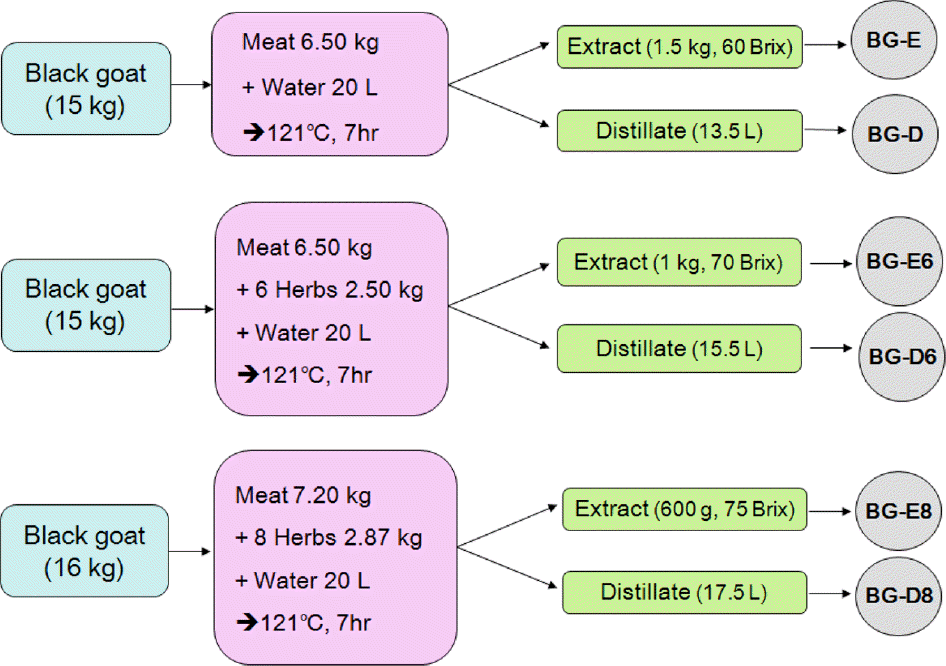
Proximate composition, volatile basic nitrogen (VBN), mineral content, free amino acid composition and free fatty acid composition in black goat meat were determined. Water extract and distillate were prepared with three groups; goat meat only (BG-E, BG-D), six herbs added group (BG-E6, BG-D6), and eight herbs added group (BG-E8, BG-D8). Osteoblast proliferation, mineralization and calcium uptake activity of MG-63 cells were measured and tartrate resistant acid phosphatase activity of osteoclasts was analyzed.
Results:
Black goat meat had remarkably low fat and high level of calcium. Glutamic acid was the most abundant amino acid. Herbs added extract groups (BG-E6 and BG-E8) showed increased MG-63 cell proliferation in a concentration dependent manner, while all the distillates did not show the effect. All extracts and distillates showed significantly increased osteoblast mineralization depending on the concentration. In particular, herb added extract, BG-E6, increased 170.3% of control and the distillate of BG-D and BG-D6 increased up to 168.5% and 159.8%, respectively. Calcium uptake activities of all water extracts showed remarkable increase of BG-E6 and BG-E8 up to 615.5% and 628.1% of control, respectively. Ditillates had no effect except BG-D6. All water extracts significantly reduced the activity of tartrate-resistant acid phosphatase (TRAP) in osteoclasts derived from mouse bone marrow.
Go to : 
REFERENCES
1.Gil B., Song H. Establishment of quality index on the black-goat meat extracts. Korean J Food Nutr. 2001. 14(4):322–328.
2.The Kyunghyang Shinmun [Internet]. Seoul: The Kyunghyang Shinmun;1993. Sep 25 [cited 2015 Jan 20]. Available from:. http://newslibrary.naver.com/viewer/index.nhn?publishDate=1993-09-25&officeId=00032&pageNo=1.
3.Yonhap News [Internet]. Seoul: Yonhap News;1996. Jul 27 [cited 2015 Jan 21]. Available from:. http://news.naver.com/main/read.nhn?mode=LSD&mid=sec&sid1=101&oid=001&aid=0004031653.
4.Choi SH., Park BY., Cho YM., Choi CY., Kwon EG., Kim YK., Hur SN. Effects of feeding browses on growth and meat quality of Korean native goats. J Anim Sci Technol. 2003. 45(5):819–824.
5.Choi SH., Kim SW., Park BY., Sang BD., Kim YK., Myung JH., Hur SN. Effects of dietary crude protein level on growth and meat quality of Korean native goats. J Anim Sci Technol. 2005. 47(5):783–788.
6.Kim SW., Yoon SH., Kim JH., Ko YG., Kim DH., Kang GH., Kim YS., Lee SM., Suh SW. Effects of feeding levels of concentrate on the growth, carcass characteristics and economic evaluation in feeds based on rice-straw of Korean black goats. J Korean Soc Grassl Forage Sci. 2012. 32(4):429–436.

7.Hwangbo S., Choi SH., Kim SW., Son DS., Jeon BS., Lee SH., Jo IH. Effects of different grazing types of hilly pasture on growth and meat quality in organic Korean black goats. Korean J Org Agric. 2008. 16(3):309–320.
8.Kim YB., Jeon KH., Lee NH., Yang SY., Moon BY., Jang A. Nutritional quality of black goat meat. Proceedings of 36th Conference of Korean Society for Food Science of Animal Resources. 2005. Oct 28; Korea University. Seoul: Korean Society for Food Science of Animal Resources; 2005.
9.Kim JS., Kim KP., Lee MC. Mineral contents and fatty acid composition of jemsosojoo. J Korean Soc Food Sci Nutr. 1998. 27(2):220–226.
10.Park CI., Kim YJ. Composition in amino acid and changes in protein, mineral contents during storage of black goat extracts. J Korean Soc Food Sci Anim Resour. 2000. 20(4):257–263.
11.Young HT., Kim MW., Choi HJ. Studies on the characterization of black goat meat and bone beverage containing honey with red ginseng. Korean J Food Nutr. 2005. 18(2):135–139.
12.Jo KS. A study on the extraction time and component analysis of goat meat with bone extract. Korean J Food Preserv. 2002. 9(4):396–399.
13.Kim SA. The recipe standardization and nutrient analysis of broiled black goat meat. Korean J Diet Cult. 2001. 16(4):269–275.
14.Kim C., Ha H., Kim H., Lee JH., Song KY. Pueraria lobata Ohwi as an osteoporosis therapeutics. Korean J Food Sci Technol. 2002. 34(4):710–718.
15.Lee Y., Lee HS., Jang SJ., Song JH. Effect of water extract of Schisandra Chinensis on osteoclast differentiation. Korean J Orient Physiol Pathol. 2010. 24(5):848–853.
16.Im KJ., Choi KH., Jung EH., Yoo DY. The effect of dried roots of Rehmannia glutinosa extract on osteoblast in rat fetus calvarial cells. J Orient Obstet Gynecol. 2013. 26(3):33–43.

17.Kim JH., Ki JY., Ann JY., Park HJ., Kim HJ., Kwak HB., Oh JM., Kim YK. Inhibitory effects of Achyranthis Bidentatae Radix on osteoclast differentiation and bone resorption. Korean J Herbol. 2010. 25(1):65–74.
18.Kwak HB., Kim JH., Kwon YM., Oh JM., Kim YK. Effect of water extract of deer antler in osteoclast differentiation. Korean J Orient Physiol Pathol. 2008. 22(4):891–895.
19.Kim YK., Choi YH., Song JH., Jang SJ., Kim HJ., Lee CH., Ahn SH., Lee JE., Kim JJ., Choi MK. Inhibitory effect of deer antler on osteoclastic bone resorption. Korean J Orient Physiol Pathol. 2009. 23(6):1299–1304.
20.Choi KH., Yoo DY. The effect of Guibi-tang water extract on osteoclast differentiation and osteoblast proliferation. J Orient Obstet Gynecol. 2014. 27(3):12–27.

21.Jeon MH., Kim M. Effect of Hijikia fusiforme fractions on proliferation and differentiation in osteoblastic MC3T3-E1 cells. J Life Sci. 2011. 21(2):300–308.

22.Koo HJ., Sohn EH., Kang SC. The optimal combination of the mixture of unripe Rubus coreanus and Astragalus membranaceus in the activation and differentiation of osteoblastic cells. Korean J Plant Resour. 2013. 26(5):658–662.

23.Im NK., Kim HJ., Kim MJ., Lee EJ., Kim HI., Lee IS. Effects of medicinal herb extracts on osteoblast differentiation and osteoclast formation. Korean J Food Sci Technol. 2010. 42(5):637–642.
24.Association of Official Analytical Chemists. Official methods of analysis. 15th edition.Washington, D.C.: Association of Official Analytical Chemists;1990.
25.Ministry of Food and Drug Safety (KR). 11. Processed meat and egg products. In: Korea Food Code. Article 5. Standards and Specifications for Each Food Product. Cheongju: Ministry of Food and Drug Safety;2009. p.37.
26.Song HN., Gil B. Analysis of nutritional composition and phenolic compound in propolis collected from falseacacia and chestnut tree in Korea. Korean J Food Sci Technol. 2002. 34(4):546–551.
27.Folch J., Lees M., Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226(1):497–509.

28.Song HN. Quality properties of fermented mugworts and the rapid pattern analysis of their volatile flavor components via surface acoustic wave (SAW) based electronic nose sensor in the GC system. Korean J Food Preserv. 2013. 20(4):554–563.

29.Song HN. Quality analysis for recycle of the drained soybean boiling water discarded in the mass production of fermented soy foods. Korean J Food Cookery Sci. 2013. 29(5):525–531.

30.Kim HK., Kim MG., Leem KH. Effects of egg yolk-derived peptide on osteogenic gene expression and MAPK activation. Molecules. 2014. 19(9):12909–12924.

31.Arpornmaeklong P., Brown SE., Wang Z., Krebsbach PH. Phenotypic characterization, osteoblastic differentiation, and bone regeneration capacity of human embryonic stem cell-derived mesenchymal stem cells. Stem Cells Dev. 2009. 18(7):955–968.

32.Miyawaki A., Llopis J., Heim R., McCaffery JM., Adams JA., Ikura M., Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997. 388(6645):882–887.
33.Kil JS., Kim MG., Choi HM., Lim JP., Boo Y., Kim EH., Kim JB., Kim HK., Leem KH. Inhibitory effects of Angelicae Gigantis Radix on osteoclast formation. Phytother Res. 2008. 22(4):472–476.

34.Hotokezaka H., Sakai E., Kanaoka K., Saito K., Matsuo K., Kitaura H., Yoshida N., Nakayama K. U0126 and PD98059, specific inhibitors of MEK, accelerate differentiation of RAW264.7 cells into osteoclast-like cells. J Biol Chem. 2002. 277(49):47366–47372.

36.Lee JW., Kim DK. Analyses of the expression profiles of genes responsible for the growth of osteoblast upon Velvet antlers treatment. J Korean Orient Pediatr. 2002. 16(1):39–74.
Go to : 
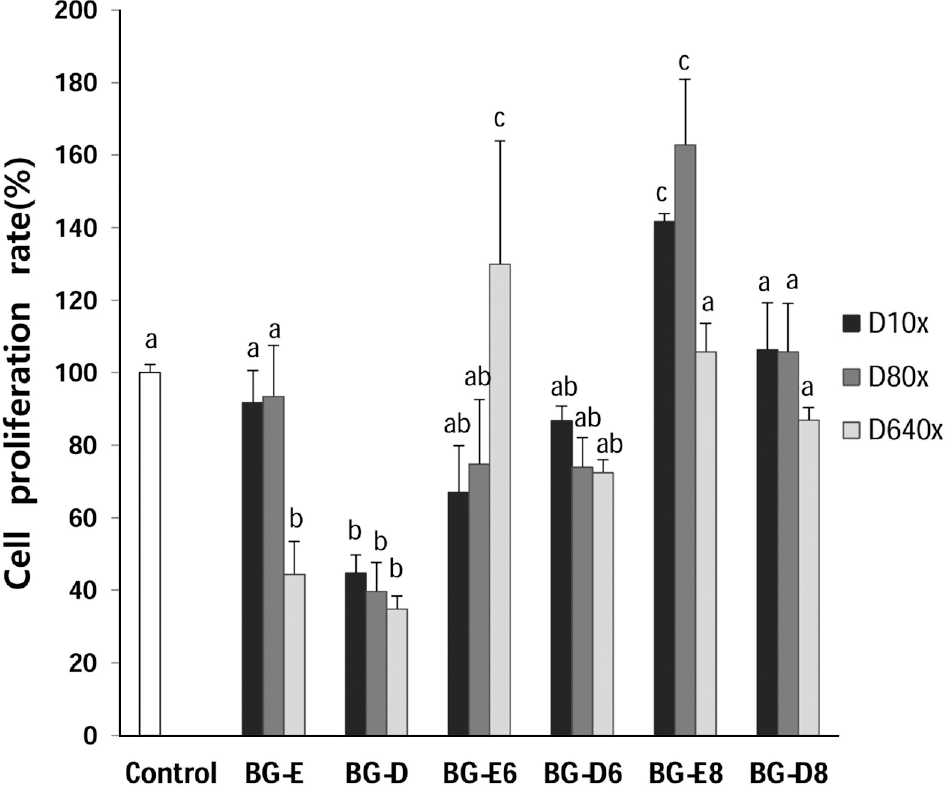 | Fig. 2.Effect of black goat meat and medicinal herb mixture on proliferation of human osteoblast MG-63 cells. D10x, 1/10 dilution; D80x, 1/80 dilution; D640x, 1/640 dilution. Data shown are the mean and SD values of three independent samples. The treatments sharing a common alphabet are not statistically significant at 5% level. |
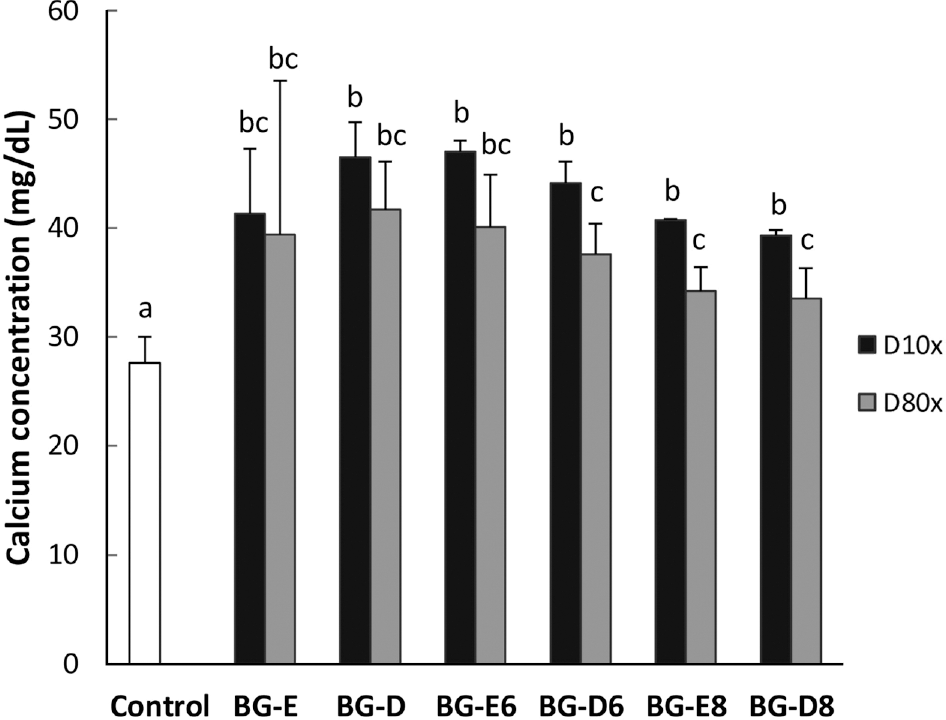 | Fig. 3.Effect of black goat meat and medicinal herb mixture on mineralization of human osteoblast MG-63 cells. D10x, 1/10 dilution; D80x, 1/80 dilution. Data shown are the mean and SD values of three independent samples. The treatments sharing a common alphabet are not statistically significant at 5% level. |
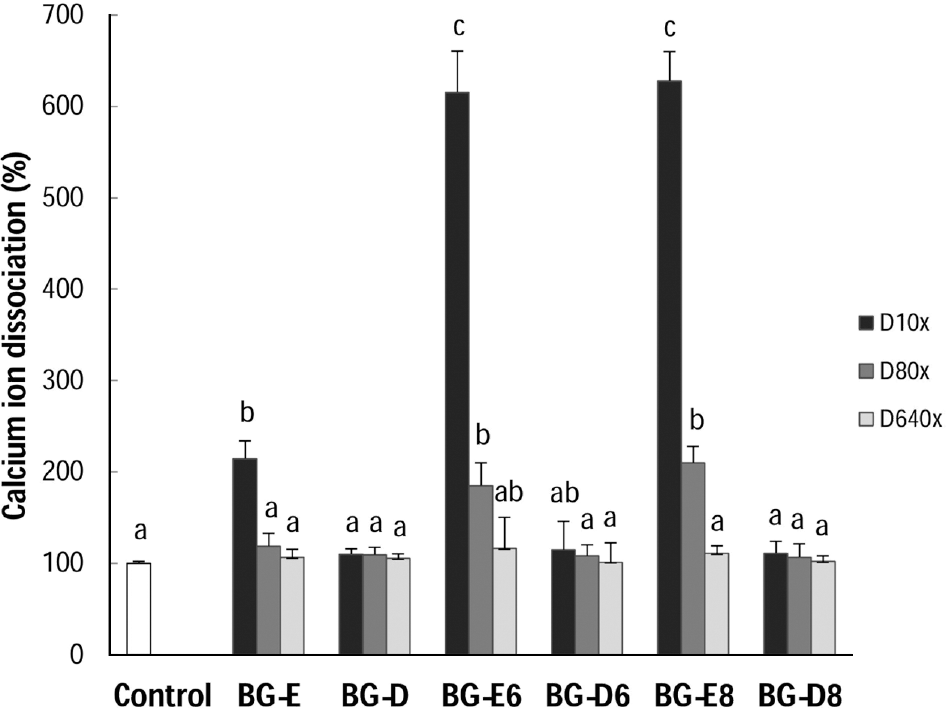 | Fig. 4.Effect of black goat meat and medicinal herb mixture on calcium uptake of human osteoblast MG-63 cells. D10x, 1/10 dilution; D80x, 1/80 dilution; D640x, 1/640 dilution. Data shown are the mean and SD values of three independent samples. The treatments sharing a common alphabet are not statistically significant at 5% level. |
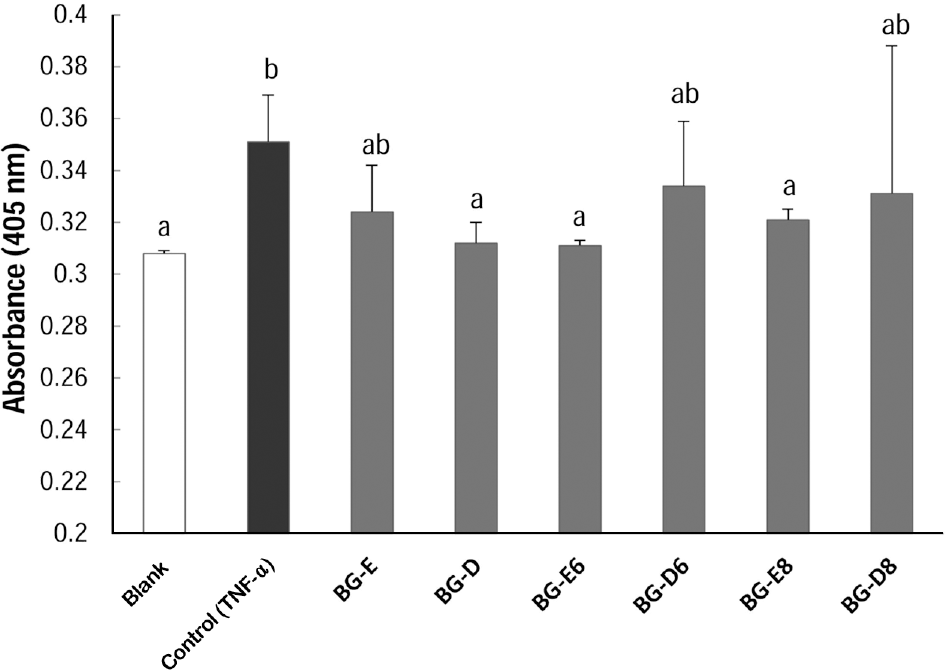 | Fig. 5.Effect of black goat meat and medicinal herb mixture on TRAP activity. Data shown are the mean and SD values of three independent samples. The treatments sharing a common alphabet are not statistically significant at 5% level. |
Table 1.
Proximate composition and volatile basic nitrogen (VBN of black goat meat
Table 2.
Mineral contents of black goat meat (Unit: mg%)
Table 3.
Amino acid composition of fore limb meat of black goat (Unit: mg/100 g)
Table 4.
Fatty acid composition of fore limb meat of black goat (Unit : %




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download