Abstract
Smilax china L., a native plant found in Asian countries, has several medicinal properties including antioxidant, anti-inflammatory, and anti-cancer effects. Although the root of the plant is commonly used as traditional herbal medicine in Korea and China, the medicinal properties of the leaves have not gained the same attention. In this study, we analyzed the antioxidant activity, α-glucosidase inhibitory effect and lipid accumulation inhibition effect of Smilax china L. leaf water extract (SCLE) and its solvent fractions. SCLE was fractionated by using a series of organic solvents, including ethylacetate (EA) and n-butanol (BuOH). The EA fraction had the highest total polyphenol content (440.20 ± 12.67 mg GAE/g) and total flavonoid content (215.14 ± 24.83 mg QE/g). The radical scavenging activity IC50 values of the EA fraction for 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-(3-ethylbenzthiazoline)-6-sulfonic acid (ABTS) were 0.022 mg/mL and 0.13 mg/mL, respectively. Further, SOD-like activity and reducing power values of the EA fraction were higher than those of the other fractions. However, both the α-glucosidase and lipid accumulation inhibition assays showed that the BuOH fraction (83.35 ± 4.18% at 1 mg/mL) and water extract (11.27 ± 2.67%) were more effective than the EA fraction (64.13 ± 6.35%, and 45.66 ± 7.20%). These results provide new insights into the potential anti-diabetic and anti-obesity effects of Smilax china L. leaf.
Figures and Tables
 | Fig. 2Total polyphenol content (A) and total flavonoid content (B) of SCLE fractions. Garlic acid and quercetin were used as standard compounds for the measurement of polyphenol and flavonoid contents respectively. Results are presented as Mean ± SD of three independent experiments. **: p < 0.001 as compared to the SCLE. |
 | Fig. 3SOD-like activity of SCLE fractions. SOD-like activity assay was performed by using a range of SCLE concentrations. Ascorbic acid was used as positive control. Results are presented as Mean ± SD of three independent experiments. *: p < 0.05 as compared to the SCLE. |
 | Fig. 4Anti-diabetic activity of SCLE fractions. α-Glucosidase inhibitory activity assay was performed using a range of concentrations (incubation time, 30 min)(A) and time-points (concentration, 0.25 mg/mL)(B). Acarbose was used as positive control. Results are presented as Mean ± SD of three independent experiments. *: p < 0.05, **: p < 0.001 as compared to the SCLE. #p < 0.001 as compared to the acarbose. |
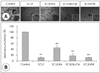 | Fig. 5Effects of SCLE fractions on lipid accumulation. 3T3-L1 adipocytes were stained with Oil-Red O for 1 hr. Stained oil droplets were dissolved in isopropanol and quantified by spectrophotometric analysis at 500 nm. Results are expressed as Mean ± SD of three independent experiments and each sample was measured in triplicate. **: p < 0.001 as compared to the control. |
References
1. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998; 21(9):1414–1431.

2. Kim SG, Choi DS. Epidemiology and current status of diabetes in Korea. Hanyang Med Rev. 2009; 29(2):122–129.
3. Choi YY, Sohn HS, Shin HT. Clinical benefits of self-monitoring of blood glucose in non-insulin treated patients with type 2 diabetes: a systematic review and meta-analysis. Korean J Clin Pharm. 2010; 20(3):183–192.
4. Toeller M. Diet therapy of diabetes mellitus. Fortschr Med. 1991; 109(2):41–42. 45
6. Boulé NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001; 286(10):1218–1227.
7. Derosa G, Maffioli P. α-Glucosidase inhibitors and their use in clinical practice. Arch Med Sci. 2012; 8(5):899–906.
8. Standl E, Schnell O. Alpha-glucosidase inhibitors 2012 - cardiovascular considerations and trial evaluation. Diab Vasc Dis Res. 2012; 9(3):163–169.

9. Heo SJ, Hwang JY, Choi JI, Han JS, Kim HJ, Jeon YJ. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent alpha-glucosidase and alpha-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol. 2009; 615(1-3):252–256.
10. Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002; 23(5):599–622.

11. Seghrouchni I, Drai J, Bannier E, Rivière J, Calmard P, Garcia I, Orgiazzi J, Revol A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus; insulin treatment efficiency. Clin Chim Acta. 2002; 321(1-2):89–96.

12. Kim BH, Son SM. Mechanism of developing diabetic vascular complication by oxidative stress. J Korean Endocr Soc. 2006; 21(6):448–459.

13. Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD. Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab. 2012; 16(6):723–737.

14. Song HS, Park YH, Jung SH, Kim DP, Jung YH, Lee MK, Moon KY. Antioxidant activity of extracts from Smilax china root. J Korean Soc Food Sci Nutr. 2006; 35(9):1133–1138.
15. Cha BC, Lee EH. Antioxidant activities of flavonoids from the leaves of Smilax china Linne. Korean J Pharmacogn. 2007; 38(1):31–36.
16. Choi SS, Cha BY, Iida K, Lee YS, Yonezawa T, Teruya T, Nagai K, Woo JT. Artepillin C, as a PPARγ ligand, enhances adipocyte differentiation and glucose uptake in 3T3-L1 cells. Biochem Pharmacol. 2011; 81(7):925–933.

17. Yadav D, Chaudhary AA, Garg V, Anwar MF, Rahman MM, Jamil SS, Khan HA, Asif M. In vitro toxicity and antidiabetic activity of a newly developed polyherbal formulation (MAC-ST/001) in streptozotocin-induced diabetic Wistar rats. Protoplasma. 2013; 250(3):741–749.

18. Kim JM, Baek JM, Kim HS, Choe M. Antioxidative and antiasthma effect of Morus bark water extracts. J Korean Soc Food Sci Nutr. 2010; 39(9):1263–1269.

19. Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71(1-2):109–114.

20. Kim KH, Kim NY, Kim SH, Han IA, Yook HS. Study on antioxidant effects of fractional extracts from Ligularia stenocephala leaves. J Korean Soc Food Sci Nutr. 2012; 41(9):1220–1225.

21. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10):1231–1237.

22. Jeong KY, Kim ML. Physiological activities of Ulmus pumila L. extracts. Korean J Food Preserv. 2012; 19(1):104–109.

23. Hue SM, Boyce AN, Somasundram C. Antioxidant activity, phenolic and flavonoid contents in the leaves of different varieties of sweet potato (Ipomoea batatas). Aust J Crop Sci. 2012; 6(3):375–380.
24. Ryu HW, Lee BW, Curtis-Long MJ, Jung S, Ryu YB, Lee WS, Park KH. Polyphenols from Broussonetia papyrifera displaying potent alpha-glucosidase inhibition. J Agric Food Chem. 2010; 58(1):202–208.
25. Jeong HJ, Lee SG, Lee EJ, Park WD, Kim JB, Kim HJ. Antioxidant activity and anti-hyperglycemic activity of medicinal herbal extracts according to extraction methods. Korean J Food Sci Technol. 2010; 42(5):571–577.
26. Lee JM, Park JH, Chu WM, Yoon YM, Park E, Park HR. Antioxidant activity and alpha-glucosidase inhibitory activity of stings of Gleditsia sinensis extracts. J Life Sci. 2011; 21(1):62–67.

27. Kim MA, Son HU, Yoon EK, Choi YH, Lee SH. Comparison of anti-diabetic activities by extracts of grape cultivar. Korean J Food Preserv. 2012; 19(3):400–405.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download