Abstract
Although the functional ingredient has been evaluated by the Korea Food and Drug Administration (KFDA) based on scientific evidence, the levels of scientific evidence and consistency of the results might vary according to emerging data. Therefore, periodic reevaluation may be needed for some functional ingredients. In this study, we reevaluated scientific evidence for the antioxidant activity of coenzyme Q10 as a functional ingredient in health functional food. Literature searches were conducted using the Medline and Cochrane, KISS, and IBIDS databases for the years 1955–2010 with the search term of coenzyme Q10 in combination with antioxidant. The search was limited to human studies published in Korean, English, and Japanese. Using the KFDA's evidence based evaluation system for scientific evaluation of health claims, 33 human studies were identified and reviewed in order to evaluate the strength of the evidence supporting a relation between coenzyme Q10 and antioxidant activity. Among 33 studies, significant effects for antioxidant activities were reported in 22 studies and their daily intake amount was 60 to 300 mg. Based on this systematic review, we concluded that there was possible evidence to support a relation between coenzyme Q10 intake and antioxidant activities. However, because inconsistent results have recently been reported, future studies should be monitored. (J Nutr Health 2013; 46(3): 218 – 225)
References
1). Korea Food and Drug Administration. Regulation for evaluation on efficacy of health functional food. Cheongwon: Korea Food and Drug Administration;2012. [cited 2012 Dec 27]. Available from:. http://www.foodnara.go.kr/hfoodi/industry/.
2). Dhanasekaran M, Ren J. The emerging role of coenzyme Q-10 in aging, neurodegeneration, cardiovascular disease, cancer and diabetes mellitus. Curr Neurovasc Res. 2005; 2(5):447–459.

3). Korea Food and Drug Administration. Specific functional food coenzyme Q10. Cheongwon: Korea Food and Drug Administration;2012. [cited 2012 Dec 27]. Available from:. http://www.foodnara.go.kr/hfoodi/industry/.
4). U.S. Food and Drug Administration. Guidance, compliance, & regulatory information. Silver Spring (MD): U.S. Food and Drug Administration;2012. [cited 2012 Dec 27]. Available from:. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/default.htm.
5). U.S. Food and Drug Administration. Qualified Health Claim Pe-tition – selenium and a reduced risk of site-specific cancers (FDA-2008-Q-0323). Silver Spring (MD): U.S. Food and Drug Administration;2009.
6). Joint FAO/WHO Codex Alimentarius Commission; Food and Agriculture Organization of the United Nations; World Health Organization. Guidelines for use of nutrition and health claims (CAC/GL 23-1997, Rev. 1–2004). Rome: Codex Alimentarius Commission;2004.
7). Hamilton SJ, Chew GT, Watts GF. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009; 32(5):810–812.

8). Dai YL, Luk TH, Yiu KH, Wang M, Yip PM, Lee SW, Li SW, Tam S, Fong B, Lau CP, Siu CW, Tse HF. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: a randomized controlled trial. Atherosclerosis. 2011; 216(2):395–401.

9). Ostman B, Sjödin A, Michaëlsson K, Byberg L. Coenzyme Q10 supplementation and exercise-induced oxidative stress in humans. Nutrition. 2012; 28(4):403–417.

10). Kon M, Tanabe K, Akimoto T, Kimura F, Tanimura Y, Shimizu K, Okamoto T, Kono I. Reducing exercise-induced muscular injury in kendo athletes with supplementation of coenzyme Q10. Br J Nutr. 2008; 100(4):903–909.

11). Singh RB, Niaz MA. Serum concentration of lipoprotein(a) decreases on treatment with hydrosoluble coenzyme Q10 in patients with coronary artery disease: discovery of a new role. Int J Cardiol. 1999; 68(1):23–29.

12). Singh RB, Wander GS, Rastogi A, Shukla PK, Mittal A, Sharma JP, Mehrotra SK, Kapoor R, Chopra RK. Randomized, double-blind placebo-controlled trial of coenzyme Q10 in patients with acute myocardial infarction. Cardiovasc Drugs Ther. 1998; 12(4):347–353.
13). Singh RB, Niaz MA, Kumar A, Sindberg CD, Moesgaard S, Littarru GP. Effect on absorption and oxidative stress of different oral Coenzyme Q10 dosages and intake strategy in healthy men. Biofactors. 2005; 25(1–4):219–224.
14). Tiano L, Belardinelli R, Carnevali P, Principi F, Seddaiu G, Littarru GP. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: a double-blind, randomized controlled study. Eur Heart J. 2007; 28(18):2249–2255.

15). Yubero-Serrano EM, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, Tasset-Cuevas I, Santos-Gonzalez M, Caballero J, Garcia-Rios A, Marin C, Gutierrez-Mariscal FM, Fuentes F, Villalba JM, Tunez I, Perez-Jimenez F, Lopez-Miranda J. Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age (Dordr). 2011; 33(4):579–590.

16). Lee BJ, Huang YC, Chen SJ, Lin PT. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition. 2012; 28(3):250–255.

17). Palomäki A, Malminiemi K, Solakivi T, Malminiemi O. Ubiqui-none supplementation during lovastatin treatment: effect on LDL oxidation ex vivo. J Lipid Res. 1998; 39(7):1430–1437.

18). Raitakari OT, McCredie RJ, Witting P, Griffiths KA, Letters J, Sullivan D, Stocker R, Celermajer DS. Coenzyme Q improves LDL resistance to ex vivo oxidation but does not enhance endothelial function in hypercholesterolemic young adults. Free Radic Biol Med. 2000; 28(7):1100–1105.

19). Singh RB, Kumar A, Niaz MA, Singh RG, Gujrati S, Singh VP, Singh M, Singh UP, Taneja C, Rastogi SS. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in patients with end-stage renal failure. J Nutr Environ Med. 2003; 13(1):13–22.

20). Kaikkonen J, Nyyssönen K, Tomasi A, Iannone A, Tuomainen TP, Porkkala-Sarataho E, Salonen JT. Antioxidative efficacy of parallel and combined supplementation with coenzyme Q10 and d-alpha-tocopherol in mildly hypercholesterolemic subjects: a randomized placebo-controlled clinical study. Free Radic Res. 2000; 33(3):329–340.
21). Chello M, Mastroroberto P, Romano R, Castaldo P, Bevacqua E, Marchese AR. Protection by coenzyme Q10 of tissue reperfusion injury during abdominal aortic cross-clamping. J Cardiovasc Surg (Torino). 1996; 37(3):229–235.
22). Chello M, Mastroroberto P, Romano R, Bevacqua E, Pantaleo D, Ascione R, Marchese AR, Spampinato N. Protection by coenzyme Q10 from myocardial reperfusion injury during coronary artery bypass grafting. Ann Thorac Surg. 1994; 58(5):1427–1432.

23). Singh RB, Niaz MA, Rastogi SS, Shukla PK, Thakur AS. Effect of hydrosoluble coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J Hum Hypertens. 1999; 13(3):203–208.

24). Gül I, Gökbel H, Belviranli M, Okudan N, Büyükbaş S, Başarali K. Oxidative stress and antioxidant defense in plasma after repeated bouts of supramaximal exercise: the effect of coenzyme Q10. J Sports Med Phys Fitness. 2011; 51(2):305–312.
25). Makhija N, Sendasgupta C, Kiran U, Lakshmy R, Hote MP, Choudhary SK, Airan B, Abraham R. The role of oral coenzyme Q10 in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2008; 22(6):832–839.

26). Singh RB, Neki NS, Kartikey K, Pella D, Kumar A, Niaz MA, Thakur AS. Effect of coenzyme Q10 on risk of atherosclerosis in patients with recent myocardial infarction. Mol Cell Biochem. 2003; 246(1–2):75–82.

27). Tiano L, Carnevali P, Padella L, Santoro L, Principi F, Brugè F, Carle F, Gesuita R, Gabrielli O, Littarru GP. Effect of Coenzyme Q10 in mitigating oxidative DNA damage in Down syndrome patients, a double blind randomized controlled trial. Neurobiol Aging. 2011; 32(11):2103–2105.

28). Kim JK, Roh SK. The effect of coenzyme Q10 supplementation on oxidative stress index and antioxidant capacity in the elderly. Korean J Exerc Nutr. 2009; 13(1):29–35.
29). Chapidze G, Kapanadze S, Dolidze N, Bachutashvili Z, Latsabid-ze N. Prevention of coronary atherosclerosis by the use of combination therapy with antioxidant coenzyme Q10 and statins. Georgian Med News. 2005; 118:20–25.
30). Migliore L, Molinu S, Naccarati A, Mancuso M, Rocchi A, Siciliano G. Evaluation of cytogenetic and DNA damage in mitochondrial disease patients: effects of coenzyme Q10 therapy. Mutagenesis. 2004; 19(1):43–49.

31). Weber C, Jakobsen TS, Mortensen SA, Paulsen G, Hølmer G. Effect of dietary coenzyme Q10 as an antioxidant in human plasma. Mol Aspects Med. 1994; 15(Suppl):S97–S102.

32). Glover EI, Martin J, Maher A, Thornhill RE, Moran GR, Tarnopolsky MA. A randomized trial of coenzyme Q10 in mitochondrial disorders. Muscle Nerve. 2010; 42(5):739–748.
33). Lee YJ, Cho WJ, Kim JK, Lee DC. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: a double-blind randomized controlled study. J Med Food. 2011; 14(4):386–390.

34). Priemé H, Loft S, Nyyssönen K, Salonen JT, Poulsen HE. No effect of supplementation with vitamin E, ascorbic acid, or coenzyme Q10 on oxidative DNA damage estimated by 8-oxo-7,8-di-hydro-2'-deoxyguanosine excretion in smokers. Am J Clin Nutr. 1997; 65(2):503–507.

35). Braun B, Clarkson PM, Freedson PS, Kohl RL. Effects of coenzyme Q10 supplementation on exercise performance, VO2max, and lipid peroxidation in trained cyclists. Int J Sport Nutr. 1991; 1(4):353–365.

36). Cooke M, Iosia M, Buford T, Shelmadine B, Hudson G, Kerk-sick C, Rasmussen C, Greenwood M, Leutholtz B, Willoughby D, Kreider R. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr. 2008; 5:8.

37). Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V. Coenzyme Q(10) improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia. 2002; 45(3):420–426.

38). Kaikkonen J, Nyyssönen K, Porkkala-Sarataho E, Poulsen HE, Metsä-Ketelä T, Hayn M, Salonen R, Salonen JT. Effect of oral coenzyme Q10 supplementation on the oxidation resistance of human VLDL+LDL fraction: absorption and antioxidative properties of oil and granule-based preparations. Free Radic Biol Med. 1997; 22(7):1195–1202.

39). Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. Eur J Clin Nutr. 2002; 56(11):1137–1142.

40). Alleva R, Tomasetti M, Battino M, Curatola G, Littarru GP, Folk-ers K. The roles of coenzyme Q10 and vitamin E on the peroxidation of human low density lipoprotein subfractions. Proc Natl Acad Sci U S A. 1995; 92(20):9388–9391.

41). Dlugosz A, Sawicka E. The chemoprotective effect of coenzyme Q on lipids in the paint and lacquer industry workers. Int J Occup Med Environ Health. 1998; 11(2):153–163.
42). Dlugosz A, Kuźniar J, Sawicka E, Marchewka Z, Lembas-Bogac-zyk J, Sajewicz W, Boratyńska M. Oxidative stress and coenzyme Q10 supplementation in renal transplant recipients. Int Urol Nephrol. 2004; 36(2):253–258.
43). Kalpravidh RW, Wichit A, Siritanaratkul N, Fucharoen S. Effect of coenzyme Q10 as an antioxidant in beta-thalassemia/Hb E patients. Biofactors. 2005; 25(1–4):225–234.
44). Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992; 1126(3):247–254.

45). Niklowitz P, Sonnenschein A, Janetzky B, Andler W, Menke T. Enrichment of coenzyme Q10 in plasma and blood cells: defense against oxidative damage. Int J Biol Sci. 2007; 3(4):257–262.

46). Sakata T, Furuya R, Shimazu T, Odamaki M, Ohkawa S, Kumagai H. Coenzyme Q10 administration suppresses both oxidative and antioxidative markers in hemodialysis patients. Blood Purif. 2008; 26(4):371–378.

47). Tomasetti M, Alleva R, Borghi B, Collins AR. In vivo supplementation with coenzyme Q10 enhances the recovery of human lymphocytes from oxidative DNA damage. FASEB J. 2001; 15(8):1425–1427.
48). Turunen M, Wehlin L, Sjöberg M, Lundahl J, Dallner G, Brismar K, Sindelar PJ. beta2-Integrin and lipid modifications indicate a non-antioxidant mechanism for the anti-atherogenic effect of dietary coenzyme Q10. Biochem Biophys Res Commun. 2002; 296(2):255–260.
49). Weber C, Sejersgård Jakobsen T, Mortensen SA, Paulsen G, Hølmer G. Antioxidative effect of dietary coenzyme Q10 in human blood plasma. Int J Vitam Nutr Res. 1994; 64(4):311–315.
Fig. 3.
Evidence table of systematic review for coenzyme Q10 and antioxidant capacity. The quality of studies was scored according to the study design, reporting of withdrawals, confounder analysis and statistical method. The scoring system used in this study was modified from KFDA and FDA scoring system (Type 1, RCT; Type 2, Cohort study; Type 3, non RCT/case-control study etc; Type 4, animal study; Type 5, in vitro study. ●: significant result in the oxidative biomarkers, ●: no significant result in the oxidative biomarkers).
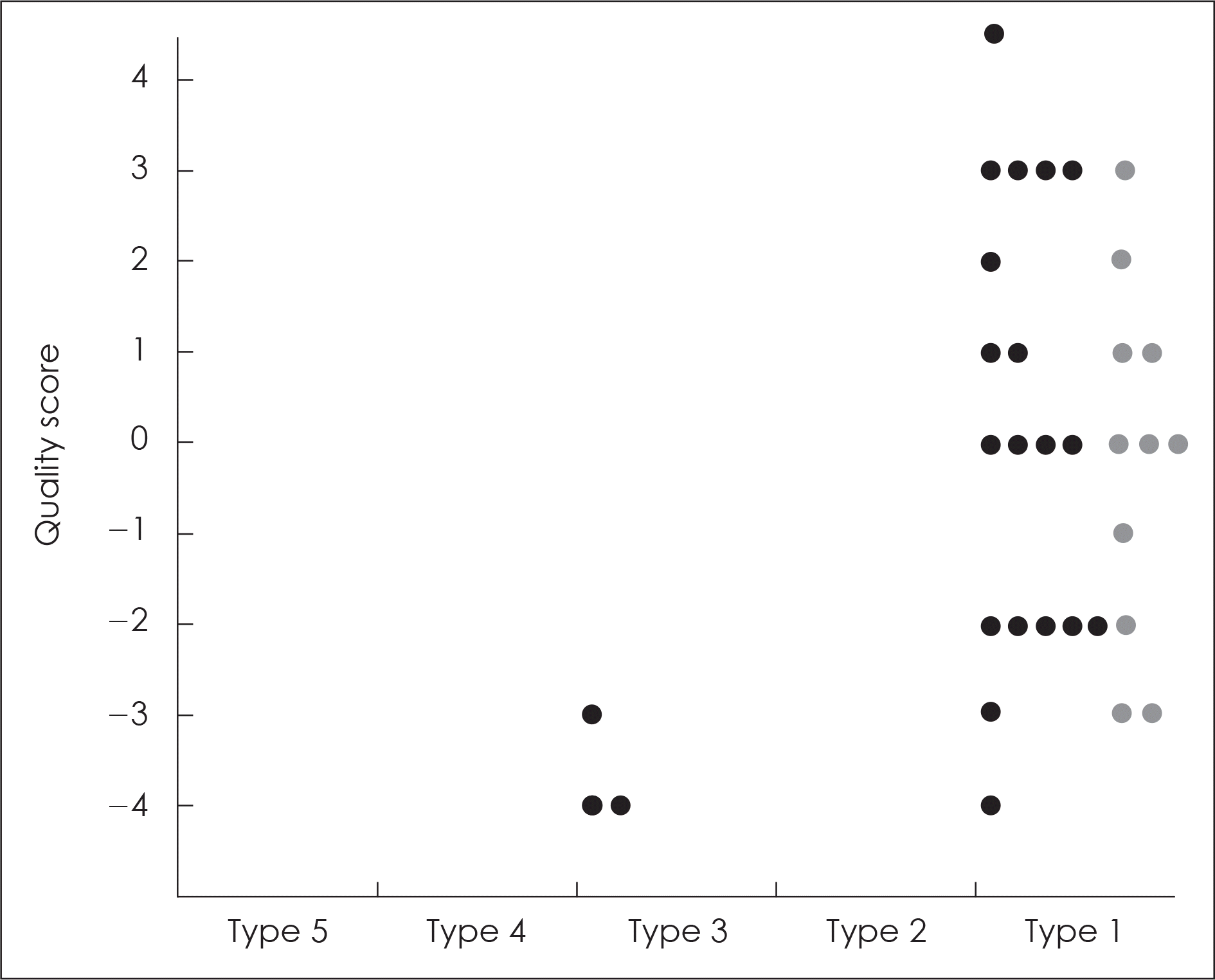
Table 1.
Characteristics of studies included for systematic review
| Ref | Study type 1) | Target | Subject no. | Dose (mg) | Result 2) | Quality score 3) |
|---|---|---|---|---|---|---|
| Hamilton et al. 20093) | RCT, DB, cross-over | Not patients | 23 | 200 | Ø | –2 |
| Dai et al. 20114) | RCT, DB, parallel | Patients | 56 | 300 | Ø | 3 |
| Ostman et al. 20125) | RCT, DB, parallel | Not patients | 25 | 90 | Ø | 2 |
| Kon et al. 200810) | RCT, DB, parallel | Not patients | 18 | 300 | + | –2 |
| Singh et al. 199911) | RCT, DB, parallel | Patients | 51 | 120 | + | 3 |
| Singh et al. 199812) | RCT, DB, parallel | Patients | 144 | 120 | + | 5 |
| Singh et al. 200513) | RCT, DB, parallel | Not patients | 60 | 100, 200 | + | –3 |
| Tiano et al. 200714) | RCT, DB, parallel | Patients | 38 | 100 | + | 3 |
| Yubero-Serrano et al. 201115) | RCT, SB, cross-over | Not patients | 60 | 200 | + | –2 |
| Lee et al. 201216) | RCT, DB, parallel | Patients | 51 | 60, 150 | + | 3 |
| Palomaki et al. 199817) | RCT, DB, cross-over | Patients | 19 | 180 | + | 3 |
| Raitakari et al. 200018) | RCT, DB, cross-over | Not patients | 12 | 150 | + | 0 |
| Singh et al. 200319) | RCT, DB, parallel | Not patients | 97 | 180 | + | 0 |
| Kaikkonen et al. 200020) | RCT, DB, parallel | Not patients | 40 | 200 | + | 1 |
| Chello et al. 199621) | RCT, DB, parallel | Not patients | 30 | 150 | + | –2 |
| Chello et al. 199422) | RCT, SB, parallel | Patients | 40 | 150 | + | –4 |
| Singh et al. 199923) | RCT, DB, parallel | Patients | 64 | 120 | + | 2 |
| Gul et al. 201124) | RCT, DB, cross-over | Not patients | 15 | 100 | + | 0 |
| Makhija et al. 200825) | RCT, parallel | Patients | 30 | 150–180 | + | –2 |
| Singh et al. 200326) | RCT, DB, parallel | Patients | 144 | 120 | + | 1 |
| Tiano et al. 201127) | RCT, DB, parallel | Not patients | 30 | 240 | + | –2 |
| Kim et al. 200928) | RCT | Not patients | 30 | 200 | + | 0 |
| Chapidze et al. 200529) | Open | Patients | 45 | 60 | + | –4 |
| Migliore et al. 200430) | Open | Not patients | 10 | 100 | + | –4 |
| Weber et al. 199431) | Open | Not patients | 22 | 90 | + | –3 |
| Glover et al. 201032) | RCT, DB, cross-over | Not patients | 30 | 1200 | Ø | –1 |
| Lee et al. 201133) | RCT, DB, parallel | Not patients | 51 | 200 | Ø | 0 |
| Prieme et al. 199734) | RCT, SB, parallel | Not patients | 142 | 90 | Ø | 1 |
| Braun et al. 199135) | RCT, DB, parallel | Not patients | 12 | 100 | Ø | 0 |
| Cooke et al. 200836) | RCT, DB, parallel | Not patients | 41 | 200 | Ø | 1 |
| Watts et al. 200237) | RCT, DB, parallel | Not patients | 40 | 200 | Ø | 0 |
| Kaikkonen et al. 199738) | RCT, SB, parallel | Not patients | 60 | 90 | Ø | –3 |
| Hodgson et al. 200239) | RCT, DB, parallel | Not patients | 80 | 200 | Ø | –3 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download