Abstract
Objective
Methods
Results
Conclusions
Figures and Tables
Figure 1
Flowchart illustrating the study design.
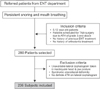
Figure 2
Simplified three-dimensional scatter plots describing the result of a cluster analysis. A, A scatter plot constructed using the factors ANB (X-axis), age (Y-axis), and FMA (Z-axis). B, A scatter plot constructed using the factors age (X-axis), ANB (Y-axis), and FMA (Z-axis). Three clusters can be identified in the three dimensions, and clusters 1, 2, and 3 are indicated by blue, red, and green dots, respectively.

Table 2
Cephalometric analysis of patients with snoring in four age groups as compared to the age-matched normative values obtained from the normal samples

Data are reported as mean ± standard deviation.
Saddle A, Angle formed between the lines Sella-Nasion and Sella-Articular; SNA, anteroposterior position of the maxilla in relation to the anterior cranial base (angle formed between the lines Sella-Nasion and Nasion-A point); Nper-A, distance between the A point and a line perpendicular to the Frankfort plane from the Nasion; SNB, anteroposterior position of the mandible in relation to the anterior cranial base (angle formed between the lines Sella-Nasion and Nasion-B point); Nper-B, distance between the B point and a line perpendicular to the Frankfort plane from the Nasion; Nper-Pog, distance between the Pogonion and a line perpendicular to the Frankfort plane from the Nasion; ANB, mathematical difference between the SNA and SNB; SUM, sum of the angles Saddle-Articular-Gonial; Articular A, angle formed between the lines Sella-Articular and Articular-Gonion; FMA, angle between the Frankfort plane and mandibular plane; PFH/AFH, ratio of posterior facial height relative to anterior facial height (S-Go/N-Me); Gonial A, angle formed between the lines Articular-Gonion and mandibular plane; MP-H, perpendicular distance of the hyoid point from the mandibular plane; H-PTV, distance of the hyoid point to the vertical pterygoid; H-C3Me, perpendicular distance of the hyoid point from the C3-Menton line; ad1, distance from the PNS to the nearest adenoid tissue measured along the line PNS-Ba; ad2, distance from the PNS to the nearest adenoid tissue measured along the line through the PNS perpendicular to the Sella-Basion; SPAS, superior posterior airway space (anteroposterior width of the pharynx measured between the posterior pharyngeal wall and the dorsum of the soft palate); MAS, middle airway space (anteroposterior width of the pharynx measured between the posterior pharyngeal wall and the palate tip); IAS, inferior airway space (anteroposterior width of the airway space along the Gonion-B point line).
Independent t-test was preliminarily performed to determine significantly different cephalometric parameters between the patients with snoring and the normal samples, for further principal component analysis; *p < 0.05, †p < 0.01, ‡p < 0.001.
Table 3
K-means cluster analysis using cephalometric principal components with the age factor
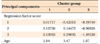
Three cephalometric principal components had been extracted from the principal component analysis, expressed as linear combinations of the FMA-articular angle as component 1, saddle angle-SNB as component 2, and MPH-ANB as component 3.
MPH, Perpendicular distance of the hyoid point from the mandibular plane.
Extraction method, principal component analysis.
Rotation method, Varimax with Kaiser normalization.
Table 4
Age-specific craniofacial patterns of the children with snoring and ATH obtained by cluster analysis

Data are reported as mean ± standard deviation or the frequency of expression.
Cluster 1 included patients aged 5–8 years with a skeletal Class I (C I) or Class II (C II) and hyperdivergent pattern; cluster 2 included patients aged 9–12 years with a skeletal Class II and hyperdivergent pattern; and cluster 3 included patients aged 7–8 years with a skeletal Class III (C III) and hyperdivergent pattern.
Normo, Normodivergent; Hypo, hypodivergent; Hyper, hyperdivergent.
*Significant frequency representing the characteristic pattern in each cluster, analyzed by Pearson's chi-square test (p < 0.001).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download