Abstract
Objective
The aim of this study was to compare recycled and unused orthodontic miniscrews to determine the feasibility of reuse. The comparisons included both miniscrews with machined surfaces (MS), and those with etched surfaces (ES).
Methods
Retrieved MS and ES were further divided into three subgroups according to the assigned recycling procedure: group A, air-water spray; group B, mechanical cleaning; and group C, mechanical and chemical cleaning. Unused screws were used as controls. Scanning electron microscopy, energy-dispersive X-ray spectrometry, insertion time and maximum insertion torque measurements in artificial bone, and biological responses in the form of periotest values (PTV), bone–implant contact ratio (BIC), and bone volume ratio (BV) were assessed.
Results
Morphological changes after recycling mainly occurred at the screw tip, and the cortical bone penetration success rate of recycled screws was lower than that of unused screws. Retrieved ES needed more thorough cleaning than retrieved MS to produce a surface composition similar to that of unused screws. There were no significant differences in PTV or BIC between recycled and unused screws, while the BV of the former was significantly lower than that of the latter (p < 0.05).
Orthodontic miniscrews are commonly used to reinforce anchorage, as they can be placed at various sites, do not depend on patient compliance, and can be loaded immediately.1 However, approximately 5% to 15% of miniscrews reportedly fail in clinical practice, and such failures are evidently associated with various factors including inflammation of peri-implant tissue.2345 Therefore, in order to prevent the inflammation or infection of surrounding tissues during the insertion of miniscrews, most of the commercially available orthodontic miniscrews are sealed and marketed for single use.
However, there is a growing interest in the use of recycled orthodontic miniscrews in order to reduce costs. Chung et al.6 reported that significant tip deformation and deposited debris such as calcium and phosphorus were observed in retrieved miniscrews. Noorollahian et al.78 reported that chemical cleaning with 37% phosphoric acid and 5.25% sodium hypochlorite did not affect the insertion, removal, or fracture torque of retrieved miniscrews.
For the use of retrieved miniscrews to be feasible in clinical practice, their surface properties and in vivo biomechanical stability after recycling should be comparable to that of unused miniscrews. However, to the best of our knowledge few studies have investigated changes in the surface morphology and chemical composition of retrieved miniscrews depending on the cleaning method used, or evaluated the in vivo biomechanical stability of retrieved miniscrews in comparison with that of unused miniscrews.
Therefore, in the present study we assessed the surface properties of retrieved orthodontic miniscrews (both those with machined surfaces [MS] and those with etched surfaces [ES]) after mechanical and chemical cleaning. We also compared the mechanical characteristics and biological responses of recycled and unused miniscrews in order to determine the feasibility of reuse from the biomechanical aspect. We hypothesized that the surface morphology and chemical composition of retrieved miniscrews after recycling would differ depending on the cleaning methods used and the type of initial surface treatment (MS vs. ES), and that there would be no significant difference in the biomechanical stability of retrieved and unused miniscrews in beagle dogs.
Four 12-month-old male beagle dogs weighing approximately 10 kg were used. Each surgical procedure was approved by the Animal Care and Use Committee of Yonsei Medical Center, Seoul, Korea. All procedures were performed under general anesthesia. Following pre-anesthetic injection of intramuscular medetomidine 10 µg/kg and subcutaneous atropine 0.05 mg/kg, alfaxalone 2 mg/kg was intravenously administered for general anesthesia. Intravenous cefazolin 20 mg/kg and intravenous or subcutaneous ketorolac 0.5 mg/kg were administered for postoperative infection control and analgesia, respectively. After the final experiment, euthanasia was induced by the injection of KCl 1–2 mol/kg after the induction of general anesthesia as described above.
Self-drilling orthodontic miniscrews (diameter 1.4 mm, length 6 mm) were used. Screws with MS (OSSH1406; Osstem Implant, Busan, Korea) and ES (OSSH1406HE; Osstem Implant) were distinguished (Figure 1). To simulate initial use, orthodontic miniscrews were placed in the maxilla (Figure 2). The right and left sides held the MS and ES groups, respectively. The buccal placement site was between the roots of the second, third, and fourth premolars and the first molar (total four), while the palatal placement site was the deepest area between the rugae, 2 mm from the mid-palatal region at the third and fourth premolars. Fourteen screws (7 MS, 7 ES) were placed in each animal (n = 56 for four animals). The screws were maintained for 4 weeks with a soft diet before retrieval (Figure 3).
All screws were retrieved by a reverse turn of the engine driver. The MS and ES groups were further divided into three subgroups (A, B, C) according to the assigned recycling procedure (Figure 4). In group A (4 MS, 4 ES), screws were cleaned with an air-water spray and rinsed with distilled water. In group B (4 MS, 4 ES), the surfaces were mechanically cleaned using an air-flow device (Air-flow® handy 2+; EMS, Nyon, Switzerland). The air-flow tip was placed 1 cm from the screw surface, and cleaning was performed for 10–15 seconds until all remnants were removed as observed by the naked eye. The screws were then rinsed in an ultrasonic bath (SHB-1025; Saehan Sonic, Seoul, Korea) with distilled water for 15 minutes. In group C (20 MS, 20 ES), screws were chemically cleaned after mechanical cleaning. Mechanically cleaned screws were thoroughly dried, completely immersed in 37% phosphoric acid (Dentto-Etch 37; Mediclus Co, Seoul, Korea) for 10 minutes, irrigated with distilled water then dried, immersed in 6% sodium hypochlorite (RC Cleaner; Il-Chung Dental Co., Seoul, Korea) for 15 minutes, then rinsed in an ultrasonic bath with distilled water for 15 minutes. All 56 recycled screws were dried, sealed in individual autosealing envelopes, sterilized at 121oC and 18 psi (124,106 Pa) in an autoclave for 20 minutes in accordance with the manufacturer's guidelines (STP-103; Hanshin Medical Co., Incheon, Korea). Group D included 40 unused, autoclaved screws (20 MS, 20 ES) as controls.
To assess changes in surface properties after recycling, we performed field emission scanning electron microscopy (SEM; JSM-7001F, JEOL, Tokyo, Japan) with a 15-kV accelerating voltage. Samples were not coated. Energy-dispersive X-ray spectroscopy (EDS) was performed to evaluate the surface elemental composition at the deepest area between threads in the mid-portion of screws. The percentage of each element was analyzed using the EDS system software (AZtecEnergy analysis software; Oxford Instruments PLC, Oxon, UK).
Five unused and five recycled screws each from the MS and ES groups were inserted for measurements using a driving torque tester (Biomaterials Korea Inc., Seoul, Korea) (Figure 5). A 500 g load was added to the 1.14 kg load of the torque tester. Screws were inserted at a uniform speed of 3 rpm in accordance with the American Society for Testing and Materials F543-02 guidelines. To standardize the insertion conditions, an artificial bone block (Sawbones; Pacific Research Laboratories Inc., Vashon Island, WA, USA) with 1-mm-thick cortical bone was used (Table 1), and intervals between adjacent screws were less than 10 mm. During insertion, the torque was measured every 0.1 second using a computer program (QuickDataAcq, Data Translation; SDK Developer, London, UK), and changes in values according to the insertion time were plotted on a graph (Figure 5). The insertion time (time from screw tip perforation of the cortical bone to initiation of cancellous bone penetration) and the maximum insertion torque (maximum torque value from the beginning to the end of screw insertion) were measured.
Four recycled screws from group C (2 MS, 2 ES) and 4 unused screws (2 MS, 2 ES) were placed on the same day in the mandibles of each dog (4 dogs, a total of 32 miniscrews, right side ES, left side MS). Recycled screws were placed between the second and fourth premolar roots and unused screws between the third premolar and first molar roots. A soft diet was provided to the animals. Two were sacrificed at 4 weeks after placement of the miniscrews, and two at 8 weeks after placement. The mechanical stability of each screw was measured three times both on the day of insertion and on the day of animal sacrifice using the periotest (periotest value, PTV) (Siemens AG, Munich, Germany).
The mandible was separated and carefully sectioned at the second, third, and fourth premolar and first molar regions to avoid damage to the screw insertion site. Each tissue block was prepared for histometric measurement via a routine procedure. Hematoxylin and eosin staining was performed for histomorphometric analyses. Each slide was analyzed using light microscopy (BX50; Olympus, Tokyo, Japan) under ×50 magnification. Histometric measurements were obtained using an image analysis program (Image-Pro Plus®; Media Cybernetics, Silver Spring, MD, USA).
The bone–implant contact ratio (% BIC; length of bone tissue in direct contact with the screw/total screw surface length from the outermost cortical bone to the third uppermost thread within the bone) and bone volume ratio (% BV; percentage bone area/total area between the imaginary line connecting the top of the thread and the screw surface from the outermost cortical bone to the third uppermost thread within the bone) were measured (Figure 6).
All statistical analyses were performed using IBM SPSS Statistics ver. 20.0 software (IBM Co., Armonk, NY, USA). Two-way ANOVA was used for insertion time and maximum insertion torque measurements in artificial bone, and linear mixed model analysis was used for PTV, BIC, and BV assessments. A p-value of < 0.05 was considered statistically significant.
SEM observation of the lateral side of orthodontic miniscrews showed that surface remnants were remarkably reduced by each recycling process, without any morphological changes such as torsion or thread deformation (Figures 7 and 8). All screws (MS and ES) in group A showed remnants as a black stain. These stains were remarkably reduced in group B, the decrease being greater for MS (Figure 7) than for ES (Figure 8). In group C, few or no stains were observed, with surface conditions similar to those of unused screws.
SEM observation of screw tips showed various degrees of damage such as blunting or deformation in all recycled screws, whereas sharp tips were observed in group D (Figure 9). Surface remnants appearing as stains showed trends similar to those observed for the lateral sides.
A large number of organic (carbon, oxygen) and inorganic elements (calcium, phosphorus) were detected by EDS analysis in both MS and ES screws in group A, but not in group B. In group B, elements such as oxygen, calcium, and phosphorus were not detected in the MS group, and the amount of carbon was similar to that in unused screws. Group C (both MS and ES) showed a surface composition similar to that of unused screws (Table 2).
All 10 (5 MS, 5 ES) unused screws were successfully inserted in artificial bone, whereas 2 recycled MS and 2 recycled ES failed to penetrate the cortical bone (40% failure rate). The successfully inserted recycled screws exhibited similar insertion times and maximum insertion torque values (Table 3).
According to the healing period, PTV showed an increasing tendency, with the increase at 8 weeks showing statistical significance (Table 4). Both BIC and BV were significantly higher at 4 weeks than at 8 weeks. The % BIC and % BV were higher in the ES groups than in the MS groups, although the differences were not significant. The BV of unused screws was significantly higher than that of recycled screws, whereas the BIC did not differ significantly (Table 4).
Previous studies on recycling of orthodontic miniscrews have mostly assessed clinically used screws with varying placement sites, durations of use, applied force, and storage periods after retrieval. In this study, in order to minimize the effects of these factors on the retrieved miniscrews, we inserted unused miniscrews in the maxillae of four beagle dogs with similar characteristics and maintained them for 4 weeks before retrieval. Furthermore, previous studies have mostly assessed miniscrews with MS. However, the use of miniscrews with treated surfaces is gradually increasing, with improved success rates.910 Therefore, we assessed both MS and ES in the present study.
In order to be suitable for reuse, retrieved screws should be infection-free, and they should have mechanical characteristics and yield biological responses similar to those of unused screws. To eliminate the risk of infection, screw surface remnants must be thoroughly removed. The recleaning and resterilization of used titanium screws alters their surface properties.11 Cleaning can be mechanical and/or chemical. For mechanical cleaning, retrieved screws were subjected to air-flow cleaning with glycine powder in this present study. Previous studies have reported that air-flow cleaning with glycine powder causes minimal damage to screw surfaces and inhibits bacterial recolonization for a short duration.121314 Following air-flow cleaning, screws are rinsed in an ultrasonic bath with distilled water for 15 minutes to disrupt the residual debris.11
For chemical cleaning, we used 37% phosphoric acid and 6% sodium hypochlorite, which are commonly used in clinics, is cheap, and easy to manipulate. The former is commonly used to etch enamel surfaces and is effective in the removal of inorganic materials from screw surfaces.815 The latter is commonly used in root canal therapy and is effective in the removal of organic materials.816 Accordingly, both chemicals together remove organic and inorganic materials without damaging the titanium surface and are considered effective for titanium screw recycling.8
The last step of recycling is sterilization, whereby the reproduction of microorganisms such as bacteria, spores, and fungi is eliminated or prevented11 and protein remnants are denatured to decrease the risk of allergenicity.8 The methods used include autoclaving, gamma irradiation, oxygen plasma treatment, and ultraviolet radiation. We used autoclaving, the most common method in dental clinics, in the present study.
SEM observations showed that MS, but not ES, can be effectively cleaned by mechanical procedures alone. Sahrmann et al.13 reported that the cleaning efficacy for titanium implant surfaces differs according to the angulation of the air-flow device. In that report, a 90° angulation was considered most effective. However, an ES is irregular, and it is impossible to apply the air-flow device at 90° to the entire surface. Therefore, more surface remnants are left in ES than in MS. On the other hand, in the present study, chemically cleaned MS and ES showed surface properties similar to those of unused screws (group D).
We clarified our SEM findings with EDS analysis. Orthodontic miniscrews are primarily fabricated from Ti6Al4V alloy. Therefore, elements in the titanium alloy other than titanium, vanadium, and aluminum indicate screw surface contamination. Carbon and oxygen are the base elements of organic molecules and are derived from contact with biological fluids, whereas phosphorus and calcium are the base elements of inorganic molecules and are derived from screw surface contact with blood or selective osseointegration islets.617 The most noticeable difference in surface composition between MS and ES was observed for mechanically cleaned screws. While MS showed a surface composition similar to that of unused screws, ES showed excessive surface remnants, although the remnants were less than were observed in air-water spray-cleaned screws. Mechanically and chemically cleaned screws also exhibited a surface composition similar to that of unused screws, regardless of whether they were MS or ES. On every screw surface, including control group screws, carbon was detected in addition to titanium, aluminum, and vanadium. These carbon-based contaminants probably originated from screw surface contact with air during autoclaving or laboratory procedures. Park et al.11 reported that autoclaving resulted in a greater amount of carbon-rich matter on the surface of titanium discs compared with other sterilization methods, and increased the hydrophobicity and contact angle.
The most remarkable morphological changes were detected at the screw tip area. Almost all recently developed orthodontic miniscrews are the self-drilling type. A sharp screw tip is therefore necessary for efficient cortical bone penetration.6 In the present study, while all unused screws were successfully inserted in artificial bone, 2/5 recycled MS and 2/5 recycled ES failed to penetrate the cortical bone. Considering that the vertical force, rotation speed, and bone quality were the same, insertion failure indicated severe deformation or blunting of the screw tip.
Successfully inserted recycled screws and unused screws showed similar maximum insertion torque values, which suggests that the overall screw morphology except that at the screw tip remained unchanged after recycling. In addition, there were no differences in insertion times between recycled and unused screws. If the vertical force was increased, the recycled screws with insertion failure could have penetrated the cortical bone and the insertion time could have decreased.6 However, excessive vertical force or pre-drilling can result in microcracks or thermal necrosis of cortical bone, and consequently, screw failure; therefore, vertical force should be increased carefully.1819
The mechanical stability of orthodontic miniscrews is primarily affected by mechanical interlocking in the cortical bone area.202122 Accordingly, we regulated the range of BIC measurements from the outermost cortical bone to the third uppermost thread within the bone. BV was measured from the total area between the imaginary line connecting the top of the thread and the screw surface, to limit the measurement area to as close to the screw surface as possible.
PTV increased with healing time in the present study. Both BIC and BV were significantly higher at 4 weeks than at 8 weeks. The increase in PTV and decreases in BIC and BV over time can be explained by the bone remodeling process.2324 From 1 to 8 weeks, when inflammatory reactions and bone resorption are dominant, BIC and BV decrease with a decrease in bone density, resulting in screw mobility and increased PTV.
BIC and BV were higher in the ES groups than in the MS groups, although the differences were not significant. This result was similar to that of previous studies.2526 The BV associated with unused screws was significantly higher than that associated with recycled screws, whereas there were no significant differences in BIC. This finding suggests that there are some differences between the biological responses associated with recycled and unused screws, regardless of the recycling procedure.
Further studies with larger sample sizes are required, to reduce statistical error. In addition, in vivo studies with longer healing periods and with orthodontic loading may yield different results.
The results of our study suggest that used orthodontic miniscrews can achieve a surface composition similar to that of unused screws when subjected to appropriate recycling processes, with mechanical and chemical cleaning used together yielding the best effects. However, screw tip deformation remains a concern, necessitating pre-drilling or an increase in the vertical force for cortical bone penetration. Furthermore, biological responses may differ between recycled and unused screws. Taken together, the results suggest that the reuse of recycled orthodontic miniscrews may not be feasible from the biomechanical aspect.
Figures and Tables
 | Figure 1Schematic diagrams of orthodontic miniscrews with (A) machined and (B) etched surfaces.Ø, Diameter.
|
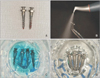 | Figure 4Images of the recycling processes used in the present study. A, Retrieved screws; B, air-flow with glycine powder (mechanical cleaning); C, immersion in 37% phosphoric acid (10 min; chemical cleaning); and D, immersion in 6% sodium hypochlorite (15 min; chemical cleaning). |
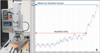 | Figure 5Assessment of mechanical characteristics. A, Photograph of the torque tester used in the study; B, graph showing insertion time and insertion torque. |
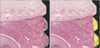 | Figure 6Measurements of (A) bone–implant contact ratio (BIC), T1 + T2 + T3 (yellow line) / T4 (blue line) and (B) bone volume ratio (BV), yellow area / area within the green line. Hematoxylin and eosin staining. |
 | Figure 7Scanning electron microscopy images of the lateral side of orthodontic miniscrews with a machined surface (×80, ×200, ×500, and ×1,000). Group A, air-water spray only and distilled water irrigation; group B, mechanical cleaning; group C, mechanical cleaning + chemical cleaning; group D, unused screw (control). |
 | Figure 8Scanning electron microscopy images of the lateral side of orthodontic miniscrews with an etched surface (×80, ×200, ×500, and ×1,000). Group A, air-water spray only and distilled water irrigation; group B, mechanical cleaning; group C, mechanical cleaning + chemical cleaning; group D, unused screw (control). |
 | Figure 9Scanning electron microscopy images of the tips of orthodontic miniscrews with (A) a machined surface and (B) an etched surface (×80, ×200). Group A, air-water spray only and distilled water irrigation; group B, mechanical cleaning; group C, mechanical cleaning + chemical cleaning; group D, unused screw (control). |
Table 2
Energy-dispersive X-ray spectroscopy analysis of the chemical composition of orthodontic miniscrew surfaces

Table 3
The mechanical characteristics of unused and recycled orthodontic miniscrews with machined or etched surfaces
References
1. Park HS, Kwon TG. Sliding mechanics with microscrew implant anchorage. Angle Orthod. 2004; 74:703–710.
2. Choi SH, Jang SH, Cha JY, Hwang CJ. Evaluation of the surface characteristics of anodic oxidized miniscrews and their impact on biomechanical stability: An experimental study in beagle dogs. Am J Orthod Dentofacial Orthop. 2016; 149:31–38.

3. Miyawaki S, Koyama I, Inoue M, Mishima K, Sugahara T, Takano-Yamamoto T. Factors associated with the stability of titanium screws placed in the posterior region for orthodontic anchorage. Am J Orthod Dentofacial Orthop. 2003; 124:373–378.

4. Cheng SJ, Tseng IY, Lee JJ, Kok SH. A prospective study of the risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004; 19:100–106.
5. Hong SB, Kusnoto B, Kim EJ, BeGole EA, Hwang HS, Lim HJ. Prognostic factors associated with the success rates of posterior orthodontic miniscrew implants: A subgroup meta-analysis. Korean J Orthod. 2016; 46:111–126.

6. Chung CJ, Jung KY, Choi YJ, Kim KH. Biomechanical characteristics and reinsertion guidelines for retrieved orthodontic miniscrews. Angle Orthod. 2014; 84:878–884.

7. Noorollahian S, Alavi S, Rafiei E. The effect of multiple processing and re-use on orthodontic mini-screw torque values. Dent Res J (Isfahan). 2015; 12:243–247.
8. Noorollahian S, Alavi S, Monirifard M. A processing method for orthodontic mini-screws reuse. Dent Res J (Isfahan). 2012; 9:447–451.
9. Choi SH, Cha JY, Joo UH, Hwang CJ. Surface changes of anodic oxidized orthodontic titanium miniscrew. Angle Orthod. 2012; 82:522–528.

10. Cho YC, Cha JY, Hwang CJ, Park YC, Jung HS, Yu HS. Biologic stability of plasma ion-implanted miniscrews. Korean J Orthod. 2013; 43:120–126.

11. Park JH, Olivares-Navarrete R, Baier RE, Meyer AE, Tannenbaum R, Boyan BD, et al. Effect of cleaning and sterilization on titanium implant surface properties and cellular response. Acta Biomater. 2012; 8:1966–1975.

12. Cochis A, Fini M, Carrassi A, Migliario M, Visai L, Rimondini L. Effect of air polishing with glycine powder on titanium abutment surfaces. Clin Oral Implants Res. 2013; 24:904–909.

13. Sahrmann P, Ronay V, Sener B, Jung RE, Attin T, Schmidlin PR. Cleaning potential of glycine air-flow application in an in vitro peri-implantitis model. Clin Oral Implants Res. 2013; 24:666–670.

14. Chairay JP, Boulekbache H, Jean A, Soyer A, Bouchard P. Scanning electron microscopic evaluation of the effects of an air-abrasive system on dental implants: a comparative in vitro study between machined and plasma-sprayed titanium surfaces. J Periodontol. 1997; 68:1215–1222.

15. Zafar MS, Ahmed N. The effects of acid etching time on surface mechanical properties of dental hard tissues. Dent Mater J. 2015; 34:315–320.

16. Kamburis JJ, Barker TH, Barfield RD, Eleazer PD. Removal of organic debris from bovine dentin shavings. J Endod. 2003; 29:559–561.

17. Eliades T, Zinelis S, Papadopoulos MA, Eliades G. Characterization of retrieved orthodontic miniscrew implants. Am J Orthod Dentofacial Orthop. 2009; 135:10.e1–10.e7. discussion 10-1.

18. Shank SB, Beck FM, D'Atri AM, Huja SS. Bone damage associated with orthodontic placement of miniscrew implants in an animal model. Am J Orthod Dentofacial Orthop. 2012; 141:412–418.

19. Yadav S, Upadhyay M, Liu S, Roberts E, Neace WP, Nanda R. Microdamage of the cortical bone during mini-implant insertion with self-drilling and self-tapping techniques: a randomized controlled trial. Am J Orthod Dentofacial Orthop. 2012; 141:538–546.

20. Youn JW, Cha JY, Yu HS, Hwang CJ. Biologic evaluation of a hollow-type miniscrew implant: an experimental study in beagles. Am J Orthod Dentofacial Orthop. 2014; 145:626–637.

21. Jung UW, Kim S, Kim YH, Cha JK, Lee IS, Choi SH. Osseointegration of dental implants installed without mechanical engagement: a histometric analysis in dogs. Clin Oral Implants Res. 2012; 23:1297–1301.

22. Eom TG, Jeon GR, Jeong CM, Kim YK, Kim SG, Cho IH, et al. Experimental study of bone response to hydroxyapatite coating implants: bone-implant contact and removal torque test. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:411–418.

23. Cha JY, Lim JK, Song JW, Sato D, Kenmotsu M, Inoue T, et al. Influence of the length of the loading period after placement of orthodontic mini-implants on changes in bone histomorphology: microcomputed tomographic and histologic analysis. Int J Oral Maxillofac Implants. 2009; 24:842–849.
24. Wei G, Hu Y, Zheng L, Huo J, Tang T, Deng F. Bone remodeling at microscrew interface near extraction site in the beagle dog mandible-histologic and immunohistochemical analyses. J Appl Oral Sci. 2013; 21:443–451.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download