INTRODUCTION
The use of orthodontic micro-implants or temporary anchorage devices for orthodontic treatment for reinforcement of anchorage without reliance on patient compliance has increased in popularity.
1 Recent studies have shown success rates of greater than 80%, however failure of micro-implants due to loss of stability is a multifactorial problem.
2 Factors that affect the success rates of micro-implants reportedly include bone quality, inflammation, and breakage of implant tips.
2
With regard to short- and long-term micro-implant stability, primary stability is influenced by the bone density of the adjacent cortical bone, screw type, and screw position, whereas extended stability is affected by new bone growth around micro-implants.
3 Partial osseointegration of micro-implants may improve stability and resistance to orthodontic force, as has been reported in various studies.
34
Zirconia has been introduced in implant dentistry as a substitute for titanium alloy due to potentially lower susceptibility to bacterial adhesion, biologically favorable tissue responses, better esthetics, and biocompatibility resulting in osseointegration.
5 It has recently been reported that zirconia showed reduced initial adhesion of microorganisms and lower bacterial colonization than titanium, which could reduce the prevalence and progression of oral infections.
6
Conventionally, zirconia implants have typically been machined with or without surface treatment, whereas a few studies have investigated surface-treated implants made using a pressure injection molding technique.
7 Recently, a novel method involving a powder injection molding (PIM) technique was reported, which uses a mold with a roughened inner surface and eliminates the need for additional surface treatment procedures to enhance the mechanical properties of the implant surface.
8
The aim of the current study was to compare the stability as insertion and removal torque and the biocompatibility of novel zirconia micro-implants made using a PIM technique with those parameters in conventional titanium micro-implants. We hypothesized that zirconia and titanium alloy micro-implants would yield comparable values as indicators of mechanical stability and clinical applicability.
DISCUSSION
Inflammation caused by micro-implants is reportedly associated with multiple factors, including level of patient hygiene, type of surrounding tissue, and micro-implant head design.
10 Notably, increased titanium ion concentrations have been found in the vicinity of titanium implants exhibiting corrosion, which results in undesirable immune reactions followed by peri-implantitis.
11 In addition, corrosion has been suggested as a confounding factor in the fracture of titanium micro-implants, based on fractographic analysis.
12 Another study has reported that titanium allergy was associated with titanium implants with a prevalence of 0.6%.
13
Recently, yttrium-stabilized tetragonal zirconia polycrystals have been introduced as a substitute for titanium implants. They exhibited inertness in the tissue and minimal ion release compared with metallic implants, in addition to higher fracture resilience and higher flexural strength.
14 Butz et al.
15 reported that zirconia abutments were comparable to titanium abutments when the survival rate, fracture strength, fracture rate, and failure mode of the abutments were evaluated after chewing simulation and fracture loading.
Bacterial adhesion studies have shown that zirconia exhibited less bacterial accumulation than titanium, with regard to both the total number of bacteria and putative pathogens.
16 Teughels et al.
17 reported that the composition and surface characteristics of the different substrates used for implant components may have direct influence on the adhesion, proliferation, and colonization of micro-organisms found in oral biofilm, affecting the pathogenesis of peri-implantitis and implant loss. Therefore, the results of the previous study support zirconium oxide as a potentially desirable micro-implant material.
In addition to material composition, the surface topography of a biomaterial is pivotal for the secondary stability of implants. Sandblasted and acid-etched surface treatment and loadings showed significant effects on the bone surrounding micro-implants, which is related to greater osseointegration and higher success rates.
18 Therefore, diverse methods of fabrication of zirconia implants have been investigated to allow the surface roughness of machined zirconia implants to improve osseointegration. Nevertheless, it has been difficult to achieve a roughened surface using conventional methods, due to the bio-inert nature and superior hardness of the material.
19
In recent years, the PIM technique was introduced to achieve surface roughness in zirconia implants as an alternative to machining methods, and it enabled simplified and economically advantageous mass production.
8 Thus, zirconia micro-implants manufactured via a PIM technique may be a comparable alternative to conventional titanium micro-implants. However, the optimal extent of roughness of zirconia micro-implants for osseointegration is still not clear.
20 Well-designed studies examining the optimal surface characteristics of zirconia micro-implants and further biomechanical and histomorphometric investigations are needed.
Among the methods for assessing implant stability, insertion torque is often measured to determine initial stability.
21 Considering that variations in micro-implant design and bone density result in a diversity of insertion torques,
21 torque tests have been conducted on a range of simulated cortical bone densities because the density of bone varies depending on its location.
9 Furthermore, removal torque has proved a reliable measure of stability, and should be comparatively high to prevent unscrewing.
22 In the current study, the MIT and MRT of zirconia micro-implants did not differ statistically significantly from those of titanium alloy micro-implants with a similar design (
Table 3). However, both groups exhibited considerably higher amounts compared with the values associated with cylindrical designs in a previous study, due to their tapered shape and surface characteristics.
23
Numerous studies have suggested that a certain range of insertion torque values is needed for the success of micro-implants.
24 In the present study, the MIT range of zirconia micro-implants was within this physiologic insertion torque range of 5 to 10 Ncm in 20 and 30 pcf biosynthetic bone;
25 however, the mean MIT in 40 pcf (10.15 Ncm) was approaching the upper limit (
Table 3). It has been suggested that a tapered zirconia micro-implant design enhances mechanical stability without an increase in microdamage, considering that microfracture is reportedly associated with greater diameter rather than with tapering of the micro-implant.
26 While a given surface treatment may contribute to increased insertion torque, it may also promote secondary stability.
18 Higher insertion torque in 40 pcf bone simulants was observed in the current study, but fracture of micro-implants was not observed. Micro-implants with small diameters are associated with several drawbacks, including fracture during insertion, and loosening due to reduced initial stability.
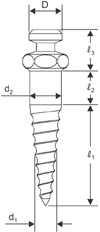
27 Thus, a diameter of 1.6 mm has been utilized in an effort to balance the risk of microdamage of cortical bone and mechanical properties required for initial stability.
26
In the current study, the lateral displacement test was used to measure the force applied to micro-implants with angulation rather than vertical force, which simulates how orthodontic forces are applied to micro-implants.
23 Regarding initial mobility, which is crucial for micro-implant success, the force was recorded when initial movement was identified at microlevels of 0.01, 0.02, and 0.03 mm.
28 Zirconia micro-implants demonstrated an ability to withstand orthodontic force, because the force required for 0.02 mm displacement exceeded 200
g , and the optimal orthodontic force range required for tooth movement is less than 200
g.
The mean compressive and tensile force required to displace zirconia micro-implants did not differ statistically significantly from that required to displace titanium alloy micro-implants, at any of the distances or angulations tested (
Tables 4 and
5). In this study, the forces for displacements of 0.01, 0.02, and 0.03 mm were recorded, which is less than the displacement used in another study that reported a critical threshold of 50 µm to 150 µm for micromovement of dental implants.
28 Thus, zirconia micro-implants can be assumed to withstand sufficient force for initial stability.
The compressive force required to displace both micro-implant groups increased gradually as the angulation increased (
Table 4). As the angulation of compression force increased, the vertical force vector directed along the long axis of the micro-implants increased and the horizontal force vector directed perpendicular to the long axis of the micro-implant decreased. This suggested more evenly distributed stress to bone simulant and increased underlying bone support against compressive force, which explained the elevated compressive force required to displace the micro-implants.
Similarly, during the application of tension force, the stress distribution became even as the angulation of the force vector increased into the implant thread and surrounding bone simulant, and more tension force was required for lateral displacement (
Table 5). Pickard et al.
29 speculated that pull-out force was greatest for orthodontic micro-implants angulated towards the direction of applied force, and gradually decreased as the lateral force vector increased, whereas micro-implants placed at an angle opposing the applied force showed the least pull-out force.
In the experimental animal study, the mean BIC of prototype zirconia micro-implants was 56.88 ± 6.72%, which is higher than that of titanium micro-implants reported in another study.
30 Thus, the prototype zirconia micro-implants showed comparable biocompatibility characteristics to titanium micro-implants.
The results of the current study suggest that zirconia micro-implants can be used clinically, as they can withstand light orthodontic compressive and tensile forces of 150–200 g at all angles. Especially, zirconia micro-implants have the potential for clinical application in patients with poor oral hygiene, metal allergy, and high esthetic demand, and they may confer a financial advantage. Further randomized clinical trials and in vivo studies examining bone remodeling and cellular responses to zirconia micro-implants under orthodontic loading are required to confirm the findings of this mechanical study.









 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download