Abstract
Objective
Methods
Results
Figures and Tables
Figure 1
A, Cone-beam computed tomography (CBCT) image cut at levels C5, C10, C15, C20, C25, and C30. Mandibular CBCT images are digitally cut into 5-mm-thick sections parallel to the mandibular plane. B, The thickness and crosssectional area of the masseter muscle at each cut level. The arrows indicate the maximum thickness of the masseter muscles, and the indicated areas represent the cross-sectional area of the masseter muscles.
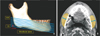
Figure 2
A, Reference points for the width and volume of the mandibular angle area; Go post (Gonion posterius), Go inf (Gonion inferius), and distobucco-occlusal point angle. B, Inter-Go post right-left (Rt-Lt) and inter-Go inf Rt-Lt widths. C, Reference points for the volume of the mandibular angle area; Go post, Go inf, distobucco-occlusal point angle, and distolinguo-occlusal point angle of second molar. D, Volume of the mandibular angle area.
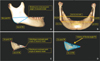
Table 1
Mean changes in masseter muscle thickness and masseter muscle cross-sectional area 6 months after botulinum toxin type A injection in groups I and II

SD, Standard deviation; C5–30, the cone-beam computed tomography images of the mandible were digitally cut into 5.0-mm-thick sections parallel to the mandibular plane; NS, non-significant.
*p<0.05, **p<0.001; Comparison of the values before and 6 months after injection(s) in each group (Wilcoxon signed-rank sum test).
†p<0.05, ††p<0.001; Comparison of the changes in thickness or cross-sectional area between groups I and II (Wilcoxon rank sum test).
Table 2
Mean changes in the width of the mandibular angle area and volume of the mandibular angle area 6 months after injection in groups I and II

SD, Standard deviation; NS, non-significant.
*p<0.05, **p<0.001; comparison of the change in values before and 6 months after injection(s) in each group (Wilcoxon signed-rank sum test).
†p<0.05, ††p<0.001; intergroup comparison of the change in values before and 6 months after injection (Wilcoxon rank sum test).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download