Abstract
Objective
Forced eruption has been proposed for the reconstruction of deficient bone and soft tissue. The aim of this study was to examine the changes in the alveolar ridge width and the vertical levels of the interproximal bone and papilla following forced eruption.
Methods
Patients whose hopeless maxillary anterior teeth were expected to undergo severe bone resorption and soft tissue recession upon extraction were recruited. In addition, patients whose maxillary anterior teeth required forced eruption for restoration due to tooth fracture or dental caries were included. Before and after forced eruption, the interproximal bone height was measured by radiographic analysis, and changes in the alveolar ridge width and the interproximal papilla height were measured with an acrylic stent.
Results
This prospective study demonstrated that the levels of the interproximal alveolar bone and papilla were significantly increased by 1.36 mm and 1.09 mm, respectively, in the vertical direction. However, the alveolar ridge width was significantly reduced by an average of 0.67 mm in the buccolingual direction. The changes in the level of the interproximal alveolar bone and papilla were positively correlated.
Conclusions
Although the levels of the interproximal bone and papilla were significantly increased, the alveolar ridge width was significantly decreased following forced eruption. There was a modest positive and significant correlation between the changes in the height of the interproximal alveolar bone and the papilla. Based on our findings, modification of vertical forced eruption should be considered when augmentation of the alveolar ridge width is required.
Advanced periodontal disease is characterized by severe attachment loss, reduction of bony support, and increased tooth mobility, often resulting in pathologic tooth migration and flaring. When the maxillary anterior teeth are seriously involved, severe esthetic problems, including a deficient alveolar ridge and papilla, may ensue following extraction. Such compromised bone and soft tissue esthetics jeopardize the outcome of the implant or fixed prosthetic treatment.1
Schropp et al.2 reported that extraction caused a substantial reduction in the alveolar ridge width and resorption of the surrounding tissues, with 50% of cases observed within the first year. Socket preservation was introduced to minimize these sequelae. Nevins et al.3 stated that this technique could control the resorption of the alveolar ridge to some degree but not completely. In an area where prosthetic restoration is scheduled, particularly for implants, the quality and quantity of the alveolar bone and gingiva are critical factors for the longevity of the implant. An inadequate quantity of buccal cortical bone often requires a bone augmentation procedure for the ideal placement of an implant and adequate thread coverage.456 Such procedures have limitations when attempting to mimic the soft tissue characteristics of the contralateral teeth. The use of connective tissue or a free gingival graft can be an option for papilla reconstruction, but the predictability of the results are low.78 Guided bone regeneration is another alternative option for hard tissue reconstruction.9 Autogenous bone is considered the gold standard, but it is difficult to harvest and the invasive surgery poses some risks.10 As an alternative non-surgical strategy, orthodontic forced eruption has been proposed as an excellent treatment option.11
Forced eruption is defined as orthodontic movement in the coronal direction through a light continuous force to induce a volumetric change in the soft tissue and bone.12 Salama and Salama13 proposed this technique for implant site development to augment the soft and hard tissue dimensions. As the papilla height facing the missing tooth area is dependent on the supracrestal fiber of the adjacent natural tooth,141516 a study by Kan et al.17 also supported this concept. The presumed level of the interproximal bone helps determine the feasible position of the post-prosthetic papilla,18 as the interproximal bone level determines the presence and vertical level of the papilla.19 Salama et al.20 reported that the enhancement of the alveolar bone height through forced eruption is effective for increasing the papilla height.
Thus far, several cases have been reported that underwent orthodontic forced eruption in the maxillary anterior teeth, but few studies have adopted a prospective design to analyze the soft and hard tissue alterations. Of particular interest, little is known about the alterations in the alveolar ridge width in the buccolingual direction resulting from orthodontic forced eruption. The present prospective study evaluated the effect of orthodontic forced eruption on the alveolar ridge width and on the vertical level of the interproximal alveolar bone and papilla.
Patients whose hopeless maxillary anterior teeth were expected to yield severe bone resorption and soft tissue recession due to chronic periodontitis upon extraction were recruited. In addition, patients whose maxillary anterior teeth required forced eruption for restorative tooth preparation due to tooth fracture or dental caries were included during a 1-year period. A total of eight subjects (five men and three women; mean age 39.75 years, range 22–53 years) were selected for the orthodontic forced eruption. Only maxillary anterior teeth that had at least a third to a fourth of the apical periodontal attachment remaining intact were included in the present study. These measurements were determined by standardized radiographs at baseline. All recruited patients were referred to the department of prosthodontics to discuss and decide upon a treatment plan before orthodontic forced eruption. This study protocol was approved by the Institutional Review Board and of Pusan National University Hospital adhered to the Declaration of Helsinki (PNUHIRB 2011083).
Before forced eruption, an inflammation control program including scaling, root planing, and instruction in oral hygiene was performed for all patients. A 0.018-inches (in) slot bracket and a 0.012-in nickel-titanium wire was configured so that a light continuous force could be applied to the tooth. A rectangular 0.018 × 0.025-in stainless steel arch wire was affixed with composites to the buccal aspect of the adjacent teeth to prevent any orthodontic movement of the anchor teeth. Intentional endodontic treatment was performed in cases where a sufficient incisal reduction was required. Every 3 weeks, an incisal reduction was performed to prevent occlusal interference resulting from the orthodontic eruption. After a 3-month eruption period, the tooth was retained for 6 weeks.
Periodontal examination included the probing depth (PD) and clinical attachment loss (CAL). All measurements were performed with a periodontal probe (Hu-Friedy, Chicago, IL, USA), and the readings were rounded up to the nearest 1 mm. The PD was measured from the gingival margin to the base of the periodontal pocket. The CAL was estimated by adding the PD measurement and the distance from the gingival margin. The PD and CAL were measured at four sites on each tooth (the mesiobuccal, midbuccal, distobuccal, and lingual sides). All parameters were measured twice at the baseline and post-treatment by a single examiner trained in periodontology. Intraclass correlation coefficients for the repeated measurements were used to present the intraexaminer reliability.
To establish a reproducible reference point for measurement, an acrylic stent was made from the diagnostic cast at baseline. A 0.018-in stainless steel wire was inserted at the incisal edge area of the tooth that was planned for forced eruption and the adjacent teeth in the stent.
With a 1-mm grid attached to the film sensor and an acrylic stent, serial standardized radiographs were taken using a paralleling technique. Although magnification errors would be expected, a customized acrylic stent with an XCP (Dentsply Rinn Corp., York, PA, USA) sensor holder was utilized for each patient. Periapical radiographs were digitalized so that the parameters were measured by pixel on the digital images using a computer program (Image J; US National Institutes of Health, Bethesda, MD, USA). A tangential line was drawn from the wire embedded in the acrylic stent to the mesial and distal peaks of the interproximal bone (Figure 1).
The gingival zenith of the target tooth was arbitrarily set as a reference point, and the distance from this point to the composite resin indentation point at the acrylic stent was recorded. This made it possible to serially measure the buccolingual ridge width at the same reference point, as the tooth is moved coronally by the forced eruption. The changes in the ridge width were calculated by comparing the buccolingual width with a caliper at the same distance before and after the forced eruption.
Using a calibrated periodontal probe, the distance from an acrylic stent to the mesial and distal peaks of the interproximal papilla was monitored before and after the forced eruption.
The data were analyzed using the SPSS statistical software program (version 20 for Windows; IBM Co., Armonk, NY, USA). The mean and standard deviation of the PD and CAL for each tooth at baseline were compared with the post-treatment values. Significance was evaluated by non-parametric Wilcoxon signed-rank test. Normality tests for the parameters of the interproximal bone height, the papilla level, and the alveolar ridge width were performed using the Kolmogorov-Smirnov test. As all parameters followed a normal distribution, parametric methods were used to analyze the data. A paired Student t-test was conducted to determine the statistical significance of the tested values. The Pearson correlation coefficient was used to seek the relationship between the changes in the interproximal bone and the papilla level. Statistical significance was accepted at a level of p < 0.05.
A total of 11 teeth from eight patients were investigated. Intraclass correlation coefficients for the measurements ranged from 0.821 to 0.982, indicating good and excellent intraexaminer reliability. After forced eruption, all clinical periodontal parameters showed changes from baseline (Table 1).
As the interproximal alveolar bone height and interproximal papillary height were measured at the mesial and distal points of the 11 teeth, respectively, the number of investigations was 22. However, as the alveolar ridge width was measured at one buccolingual point per tooth, the number of investigations was 11 (Table 2).
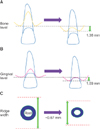
The mean interproximal alveolar bone height at the baseline and at post-treatment was 9.64 mm and 8.28 mm, respectively, demonstrating a significant increase of approximately 1.36 mm in height upon forced eruption (p < 0.05). The mean interproximal papilla height at the baseline and post-treatment was 11.25 mm and 10.17 mm, respectively, demonstrating a significant increase of 1.09 mm in height upon forced eruption (p < 0.05). The alveolar ridge width at baseline and at post-treatment was 7.42 mm and 6.75 mm, respectively, demonstrating a 0.67-mm decrease, which was statistically significant (p < 0.05, Table 2).
The level of the papilla was increased as the level of the interproximal alveolar bone increased, and this relationship reached statistical significance based on the Pearson correlation coefficient analysis (p < 0.05, Table 3).
This prospective study was designed to examine the changes in the alveolar ridge width and vertical level of the interproximal alveolar bone and papilla following a forced eruption. The success of forced eruption depends on an intact attachment apparatus spanning at least one fourth of the apical region.21 Therefore, only teeth that met the criteria were included in the study. Some authors recommend that rapid extrusions should be performed with a force higher than 50 g, whereas the maximum force for slow orthodontic forced eruption should not exceed 30 g.2223 Salama and Salama13 reported that orthodontic forced eruption assisted in the removal of deep infrabony defects and increased the probability that the implant would be placed in the ideal position. As an alternative to the surgical procedure, forced eruption may be a better choice because bone is generated from the attachment apparatus of the host.11 Celenza24 also reported that new bone formed by forced eruption had better quality than an autograft or xenograft due to osteocytes in the bone lacunae of living cells.
The vertical levels of the interproximal alveolar bone and papilla could be significantly and predictably enhanced by forced eruption, as shown in this prospective study (Figures 2 and 3). Moreover, there was a positive correlation between these two parameters. As the distance between the alveolar bone crest and the cementoenamel junction remains unchanged, the eruption of teeth gives rise to an increase in the interproximal alveolar bone level.25 The movement of the interproximal alveolar bone in the incisal direction would induce a bone peak that stimulates the corresponding development of the soft tissue peak.8 Free gingiva follows the tooth in 90% of cases, and attached gingiva follows in 80% of cases, whereas the mucogingival junction remains in the same position.26 Therefore, changes in the vertical level of the interproximal alveolar bone and papilla level may be predictable. Having the teeth move not only in the vertical direction but also in the mesial or distal directions would encourage a gain in papilla height.27 Thus, forced eruption with mesial or distal tilting forces appears to be a more appealing strategy for increasing the papilla height.
The alveolar ridge width was reduced by an average of 0.67 mm following the forced eruption, which was statistically significant. Several studies attributed the decrease in ridge width after forced eruption to the shape of a single-rooted tooth.28 The buccolingual width of a single-rooted tooth gradually becomes narrower from the cementoenamel junction to the apical end. Consequently, the eruption of a single-rooted tooth causes a narrower portion of the root to be located where a thicker root portion was in place before the forced eruption. This may lead to an esthetic concern due to an undesirable ridge contour.26 A narrower root structure may be inadequate so that the induction of osseous regeneration may be compromised in the mesiodistal or buccolingual direction.11 To overcome this problem, Zuccati and Bocchieri21 proposed orthodontic extrusion combined with buccal root torque, which may enlarge the buccolingual alveolar ridge width as an alternative to conventional vertical orthodontic eruption. This technique was suggested to achieve better alveolar ridge width in the case of insufficient buccal bone and gingiva because the width of the alveolar ridge is maintained at the middle portion as the root tip continues to erupt during forced eruption. In 2003, Nozawa et al.29 reported that forced eruption combined with buccal root torque induced buccal and coronal bone augmentation with soft tissue enlargement. The authors suggested that this technique stimulates the lingual and apical periodontal membranes for coronal and buccal bone formation around a tooth associated with severe buccal bone resorption. Therefore, forced eruption with buccal root torque would be effective for increasing the alveolar ridge width. Unfortunately, due to the limited study period and number of subjects, the present study considered only the straight vertical forced eruption technique. Further studies including forced eruption with buccal root torque are warranted to verify the progressive effect on the alveolar ridge width.
This prospective study demonstrated that the levels of the interproximal alveolar bone and papilla were significantly increased by 1.36 mm and 1.09 mm, respectively, in the vertical direction through the application of the forced eruption technique. However, the alveolar ridge width was significantly reduced by an average of 0.67 mm in the buccolingual direction. The changes in the interproximal alveolar bone level and papilla were positively correlated.
Figures and Tables
Figure 1
Schematic radiographic illustration demonstrating the measurement of interproximal alveolar bone height. Horizontal bar represents the tangential line drawn from the wire in the acrylic stent and used as a reference point.
Figure 2
Photographic images of maxillary anterior teeth before and after the forced eruption using orthodontic appliances. Marginal and interproximal soft tissue can be seen creeping toward the incisal edge (arrows).

Figure 3
Schematic drawings demonstrating the changes of the interproximal alveolar bone height (A), papilla height (B), and alveolar ridge width (C) before and after the forced eruption.
References
1. Romano R, Landsberg CJ. Reconstruction of function and aesthetics of the maxillary anterior region: a combined periodontal/orthodontic therapy. Pract Periodontics Aesthet Dent. 1996; 8:353–361.
2. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003; 23:313–323.
3. Nevins M, Camelo M, De Paoli S, Friedland B, Schenk RK, Parma-Benfenati S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006; 26:19–29.

4. Korayem M, Flores-Mir C, Nassar U, Olfert K. Implant site development by orthodontic extrusion. A systematic review. Angle Orthod. 2008; 78:752–760.
5. Tian Y, Liu F, Sun H, Lv P, Cao Y, Yu M, et al. Alveolar bone thickness around maxillary central incisors of different inclination assessed with cone-beam computed tomography. Korean J Orthod. 2015; 45:245–252.

6. Choi JH, Yu HS, Lee KJ, Park YC. Three-dimensional evaluation of maxillary anterior alveolar bone for optimal placement of miniscrew implants. Korean J Orthod. 2014; 44:54–61.

7. Carranza N, Zogbi C. Reconstruction of the interdental papilla with an underlying subepithelial connective tissue graft: technical considerations and case reports. Int J Periodontics Restorative Dent. 2011; 31:e45–e50.
8. Salama H, Salama M, Kelly J. The orthodontic-periodontal connection in implant site development. Pract Periodontics Aesthet Dent. 1996; 8:923–932. quiz 934.
9. Park YS, Yi KY, Moon SC, Jung YC. Immediate loading of an implant following implant site development using forced eruption: a case report. Int J Oral Maxillofac Implants. 2005; 20:621–626.
10. Steigmann M, Salama M, Wang HL. Periosteal pocket flap for horizontal bone regeneration: a case series. Int J Periodontics Restorative Dent. 2012; 32:311–320.
11. Uribe F, Taylor T, Shafer D, Nanda R. A novel approach for implant site development through root tipping. Am J Orthod Dentofacial Orthop. 2010; 138:649–655.

12. Stevens BH, Levine RA. Forced eruption: a multidisciplinary approach for form, function, and biologic predictability. Compend Contin Educ Dent. 1998; 19:994–998. 10001002–1004 passim.
13. Salama H, Salama M. The role of orthodontic extrusive remodeling in the enhancement of soft and hard tissue profiles prior to implant placement: a systematic approach to the management of extraction site defects. Int J Periodontics Restorative Dent. 1993; 13:312–333.
14. Greenstein G, Cavallaro J, Tarnow D. When to save or extract a tooth in the esthetic zone: a commentary. Compend Contin Educ Dent. 2008; 29:136–145. quiz 146, 158.
15. Choquet V, Hermans M, Adriaenssens P, Daelemans P, Tarnow DP, Malevez C. Clinical and radiographic evaluation of the papilla level adjacent to single-tooth dental implants. A retrospective study in the maxillary anterior region. J Periodontol. 2001; 72:1364–1371.

16. Grunder U. Stability of the mucosal topography around single-tooth implants and adjacent teeth: 1-year results. Int J Periodontics Restorative Dent. 2000; 20:11–17.
17. Kan JY, Rungcharassaeng K, Umezu K, Kois JC. Dimensions of peri-implant mucosa: an evaluation of maxillary anterior single implants in humans. J Periodontol. 2003; 74:557–562.

18. Salama M, Salama H, Garber D. Guidelines for aesthetic restorative options and implant site enhancement: the utilization of orthodontic extrusion. Pract Periodontics Aesthet Dent. 2002; 14:125–130.
19. Funato A, Salama MA, Ishikawa T, Garber DA, Salama H. Timing, positioning, and sequential staging in esthetic implant therapy: a four-dimensional perspective. Int J Periodontics Restorative Dent. 2007; 27:313–323.
20. Salama M, Ishikawa T, Salama H, Funato A, Garber D. Advantages of the root submergence technique for pontic site development in esthetic implant therapy. Int J Periodontics Restorative Dent. 2007; 27:521–527.
21. Zuccati G, Bocchieri A. Implant site development by orthodontic extrusion of teeth with poor prognosis. J Clin Orthod. 2003; 37:307–311. quiz 313.
22. Bondemark L, Kurol J, Hallonsten AL, Andreasen JO. Attractive magnets for orthodontic extrusion of crown-root fractured teeth. Am J Orthod Dentofacial Orthop. 1997; 112:187–193.

23. Reitan K. Clinical and histologic observations on tooth movement during and after orthodontic treatment. Am J Orthod. 1967; 53:721–745.

24. Celenza F. The development of forced eruption as a modality for implant site enhancement. Alpha Omegan. 1997; 90:40–43.
25. Berglundh T, Marinello CP, Lindhe J, Thilander B, Liljenberg B. Periodontal tissue reactions to orthodontic extrusion. An experimental study in the dog. J Clin Periodontol. 1991; 18:330–336.

26. Kajiyama K, Murakami T, Yokota S. Gingival reactions after experimentally induced extrusion of the upper incisors in monkeys. Am J Orthod Dentofacial Orthop. 1993; 104:36–47.

27. Kokich VG, Kokich VO. Interrelationship of orthodontics with periodontics and restorative dentistry. In : Nanda R, editor. Biomechanics and esthetic strategies in clinical orthodontics. St. Louis: Elsevier;2005. p. 348–372.
28. Potashnick SR, Rosenberg ES. Forced eruption: principles in periodontics and restorative dentistry. J Prosthet Dent. 1982; 48:141–148.

29. Nozawa T, Sugiyama T, Yamaguchi S, Ramos T, Komatsu S, Enomoto H, et al. Buccal and coronal bone augmentation using forced eruption and buccal root torque: a case report. Int J Periodontics Restorative Dent. 2003; 23:585–591.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download