Abstract
Objective
Root mobility due to reciprocating movement of the tooth (jiggling) may exacerbate orthodontic root resorption (ORR). "Jiggling" describes mesiodistal or buccolingual movement of the roots of the teeth during orthodontic treatment. In the present study, buccolingual movement is described as "jiggling." We aimed to investigate the relationship between ORR and jiggling and to test for positive cell expression in odontoclasts in resorbed roots during experimental tooth movement (jiggling) in vivo.
Methods
Male Wistar rats were divided into control, heavy force (HF), optimal force (OF), and jiggling force (JF) groups. The expression levels of cathepsin K, matrix metalloproteinase (MMP)-9 protein, interleukin (IL)-6, cytokine-induced neutrophil chemoattractant 1 (CINC-1; an IL-8-related protein in rodents), receptor activator of nuclear factor κB ligand (RANKL), and osteoprotegerin protein in the dental root were determined using immunohistochemistry.
Results
On day 21, a greater number of root resorption lacunae, which contained multinucleated odontoclasts, were observed in the palatal roots of rats in the JF group than in rats from other groups. Furthermore, there was a significant increase in the numbers of cathepsin K-positive and MMP-9-positive odontoclasts in the JF group on day 21. Immunoreactivities for IL-6, CINC-1, and RANKL were stronger in resorbed roots exposed to jiggling than in the other groups on day 21. Negative reactivity was observed in the controls.
Orthodontic root resorption (ORR) is one of the most difficult procedure-related adverse events to predict in cases of orthodontic tooth movement (OTM), and may cause permanent loss of the dental structure at the root apex. In an epidemiological study by Kaley and Phillips,1 all patients who underwent comprehensive orthodontic treatment presented root shortening, and 3% of the patients had severe root resorption (shortening by more than one-quarter of the root length) with root shortening of the maxillary central incisors. Previous studies have linked the severity of root resorption to various factors, including the type of orthodontic appliance,23 magnitude of the applied force,456 duration of force application, type of tooth movement, local and systemic diseases, patient age, genetic factors related to root anomalies, previous trauma, and ethnicity.
Orthodontists encounter ORR when teeth (especially maxillary anterior teeth) are shifted by a reciprocating movement ("jiggling") during orthodontic treatment; the generated forces move the roots of the teeth mesiodistally or buccolingually. Jiggling movements are thought to induce ORR.78 However, the influence of jiggling forces during OTM are not fully understood. Therefore, this study focused on the relationship between ORR and jiggling. In an in vivo experiment, we investigated the protein expression levels of interleukin (IL)-6, cytokine-induced neutrophil chemoattractant 1 (CINC-1; an IL-8-related protein in rodents), receptor activator of nuclear factor κB ligand (RANKL), and osteoprotegerin (OPG) during experimental jiggling tooth movement in a rat model.
The animal experimental protocol used in this study was approved by the Ethics Committee for Animal Experiments at the Nihon University School of Dentistry at Matsudo (Chiba, JAPAN) (approval number: AP13MD003). In total, 50 8-week-old male Wistar rats (body weight, 350 ± 10 g; Sankyo Labo Service, Tokyo, Japan) were used for the experiments.
The animals were anesthetized with pentobarbital sodium (40 mg/kg of body weight) for the application of orthodontic devices. Experimental tooth movement was induced using the method described by Hayashi et al.9 with a quad helix-type device (diameter: 0.012 inches [0.3048 mm], stainless steel wire; Tomy International, Inc., Tokyo, Japan) ligated to a maxillary first molar cleat with a 0.008-inches (0.2032 mm) stainless steel ligature wire (Tomy International, Inc.). The maxillary first molar was palatally or buccally moved by the appliance with a force of 10 g or 50 g (Figure 1). The force was measured in grams using a spring scale. The activated appliance was ligated to the maxillary first molar with ligature wire. The experimental period was 21 days. The rats were randomly assigned to four groups: the control group (n = 5) with no appliance; the optimal force (OF) group (n = 15), treated with 10 g of compression (palatal side of the root); the heavy force (HF) group (n = 15), treated with 50 g of compression (palatal side of the root); and the jiggling force (JF) group (n = 15), treated with 10 g of compression from day 0 to day 7 (palatal side of the root), 10 g of tension from day 7 to day 14 (buccal side of the root), 10 g of compression day 14 to day 21 (palatal side of the root) (Figure 2).
The following in vivo experiments were performed, as described by Nakano et al.10 Each sample was sliced continuously into 4-µm sections perpendicular to the long axis of the through center of pulp at distal palatal (DP) root of the maxillary first molar in the frontal cross-section, and prepared for hematoxylin and eosin (H&E) and immunohistochemical staining.9 The periodontal tissue was observed in the buccal and palatal portions of the DP root of the left first upper molar. Detailed observations were made in the A area, a section of the root 300 µm in height and 225 µm in width in the direction from the top of the alveolar bone surface on the palatal side (box A); and in the B area, a section 300 µm in height and 225 µm in width that accounted for less than 150 µm of the root in the direction of the bifurcation on the buccal side (box B), on the pressure/tension side during tooth movement (Figure 3).11 In both areas, positive cells were counted manually. The A area was on the compression side for 21 days (days 0–21) in the OF and HF groups; and in the JF group, on the compression side for the first and final 7 days (days 0–7 and 15–21) and on the tension side during the remaining 7 days (days 8–14). Conversely, the B area was on the tension side for 21 days (days 0–21) in the OF and HF groups, versus on the tension side during the first and final 7 days (days 0–7 and 15–21) and on the compression side during the remaining seven days (days 8–14) in the JF group. The control group animals did not experience any tooth movement. Frontal serial sections were generated to include 15 consecutive slides adjacent to the center of the line connecting the DP root and distal buccal root. The number of positive cells on each slide was counted after immunohistochemical staining. The mean values number of cell counts from 15 slides at the rats were used to calculate the ratio of positive cells to all cells.
The tissue sections were deparaffinized, and endogenous peroxidase activity was quenched via incubation in 3% H2O2 in methanol for 30 minutes at room temperature. After washing in Tris-buffered saline (TBS), the sections were incubated with polyclonal anti-goat cathepsin K (Santa Cruz Biotechnology, Inc., Dallas, TX, USA; working dilution, 1:100), polyclonal anti-goat matrix metalloproteinase (MMP)-9 (Santa Cruz Biotechnology; working dilution, 1:50), polyclonal anti-goat IL-6 (Santa Cruz Biotechnology; working dilution, 1:100), monoclonal anti-rat CINC-1 (American Research Products, Inc., Belmont, MA, USA; working dilution, 1:50), polyclonal anti-rabbit RANKL (Santa Cruz Biotechnology; working dilution, 1:100), and polyclonal anti-goat OPG (Santa Cruz Biotechnology; working dilution, 1:100) for 18 hours at 4℃. Cathepsin K, MMP-9, IL-6, CINC-1, RANKL, and OPG were stained using the Histofine Simple Stain MAX PO kit (Nichirei, Co., Tokyo, Japan) according to the manufacturer's protocol. The sections were rinsed with TBS and the final color reactions were performed using a 3,3'-diaminobenzidine tetrahydrochloride substrate reagent and aminoethyl carbazole. The sections were then counterstained with hematoxylin. For the immunohistochemical controls, several sections were incubated with either nonimmune rabbit immunoglobulin G or 0.01 M phosphate-buffered saline instead of the primary antibody, and negative reactivity was observed.
The body weights of the rats in each force group showed no change during the experimental period. No significant difference in body weight was observed during the experimental period between the force groups (data not shown).
Regarding areas A and B, in the control group (applied orthodontic force; 0 g) on days 7, 14, and 21 after tooth movement, the rats specimens were composed of relatively dense connective tissue fibers and fibroblasts that ran regularly in a horizontal direction from the root cementum towards the alveolar bone. Blood capillaries were mainly recognized near the alveolar bone in the periodontal ligament (PDL), and the root surfaces were relatively smooth (Figure 4a–4f).
In the A and B areas in the OF group (applied orthodontic force; 10 g), the arrangement of fibers and fibroblasts became coarse and irregular, and blood capillaries were compressed on days 7 and 14. Resorption lacunae with few multinucleated odontoclasts were observed on palatal root surfaces (Figure 4g, 4h, 4j, and 4k). On day 21, root resorption lacunae with a few multinucleated odontoclasts were observed on root surfaces (Figure 4i and 4l).
In the A area in the HF group (applied orthodontic force; 50 g), root resorption lacunae with multinucleated odontoclasts were identified on root surfaces on day 7 after the application of orthodontic force (Figure 4m). Many resorption lacunae with multinucleated odontoclasts were observed on roots on day 14 (Figure 4n). On day 21, root resorption lacunae with multinucleated odontoclasts were mostly observed on the root surface (Figure 4o). Multinucleated odontoclasts on the palatal root surface and root resorption lacunae gradually increased from day 7 through day 21. Conversely, in the B area in the HF group (applied orthodontic force; 50 g), resorption lacunae with few multinucleated odontoclasts were observed on buccal root surfaces on days 14 and 21 (Figure 4p–4r).
In the A and B areas in the JF (applied orthodontic force; 10 g) group, the arrangement of fibers and fibroblasts became coarse and irregular, and blood capillaries were compressed on day 7, as in the OF group (applied orthodontic force; 10 g). Resorption lacunae with few multinucleated odontoclasts were observed on palatal root surfaces (Figure 4s and 4v). On day 14, root resorption lacunae were increased compared to day 7 (Figure 4t and 4w). On day 21, many root resorption lacunae containing multinucleated odontoclasts were observed on the palatal roots (Figure 4u and 4x).
In the control group, no resorption lacunae with cathepsin K-positive or MMP-9-positive multinucleated odontoclasts were observed on the surfaces of the roots in either the A area or B area during the experimental period (Figures 5 and 6a–6f).
In the OF group, resorption lacunae with cathepsin K-positive and MMP-9-positive multinucleated odontoclasts were also not observed on the surfaces of roots in the A area or B area from day 7 to day 21 (Figures 5 and 6g–6l).
In the A area in the HF group, few root resorption lacunae with multinucleated cathepsin K-positive and MMP-9-positive odontoclasts were identified on root surfaces on day 7 (Figures 5 and 6m). On days 14 and 21, many root resorption lacunae with cathepsin K-positive and MMP-9-positive multinucleated odontoclasts were observed (Figures 5,6n, and 6o). In the B area, no root resorption lacunae with cathepsin K-positive or MMP-9-positive multinucleated odontoclasts were observed on day 7 (Figures 5,6p, and 6q). However, they were observed on days 14 and 21 (Figures 5,6q, and 6r).
In the JF group on day 7 after tooth movement, no resorption lacunae with cathepsin K-positive or MMP-9-positive multinucleated odontoclasts were observed on root surfaces in the A or B areas (Figures 5 and 6s). On day 14, a few cathepsin K-positive and MMP-9-positive odontoclasts were observed (Figures 5,6t, and 6w). Furthermore, on day 21, many root resorption lacunae with cathepsin K-positive and MMP-9-positive odontoclasts were observed (Figures 5,6u, and 6x).
In the control group in the A and B areas, IL-6-positive, CINC-1-positive, and RANKL-positive cells were rarely observed in PDL tissues during days 7 through 21 (Figures 7,8, and 9a–9f).
In the OF group, few IL-6-positive, CINC-1-positive, and RANKL-positive cells were observed in PDL tissues on root surfaces in the A area through day 21 (Figures 7,8, and 9g–9i). Conversely, in the OF group (10 g) in the B area, they were rarely observed in PDL tissues during days 7 through 21 (Figures 7,8, and 9j–9l).
In the HF group, few IL-6-positive, CINC-1-positive, and RANKL-positive cells were observed in PDL tissues on day 7 in the A areas of the roots, while increased numbers were observed on days 14 and 21 (Figures 7,8, and 9m–9o). Conversely, in the B area, no IL-6-positive, CINC-1-positive, or RANKL-positive cell was observed in PDL tissues on day 7, while a few cells were observed on days 14 and 21 (Figures 7,8, and 9p–9r).
In the JF group, no IL-6-positive, CINC-1-positive, or RANKL-positive cell was observed on day 7, although cells were observed on day 14, and increased numbers were observed on day 21 in both the A and B areas of the root surfaces (Figures 7,8, and 9s–9x).
In all groups, few OPG-positive cells were observed in the PDL during days 7 through 21 (Figure 10).
In our quantitative evaluations, the numbers of cathepsin K-positive and MMP-9-positive odontoclasts were found to significantly increase in the HF and JF groups on day 21 versus the control group in both the A and B areas (p < 0.01). Furthermore, their numbers were significantly increased in the JF group on day 21 compared with the HF group (p < 0.01). There was no significant difference in the A or B areas between the OF group and the control group (Figure 11A and 11B).
The numbers of IL-6-positive, CINC-1-positive, and RANKL-positive cells were found to increase significantly in the HF and JF groups on day 21 versus the control group in both the A and B areas. Their numbers were greater in the JF group than in the HF group in both the A and B areas on day 21 (p < 0.01) (Figure 11C–11E). More significant differences were observed in the B area than in the A area (Figure 11C–11E). The number of OPG-positive cells was significantly ncreased in the OF, HF and JF group on day 21 compared with the control group; however, there was no significant difference between OF, HF and JF group (Figure 11F).
All methods in this study, including the application of a 10-g light force, 50-g heavy force, and 10-g jiggling force, produced tooth movement over a period of 21 days in rats. The apparatuses did not affect the growth of the rats. Gonzales et al.12 showed that the application of a 10-g light force produced significantly greater tooth movement with significantly less root resorption over 28 days versus a greater force in rats. The optimum force for moving rat upper molars may be even less than 10 g, as previously suggested.13 Many investigators have reported that root resorption is aggravated by increased force magnitudes.613 For example, Gameiro et al.14 demonstrated osteoclastic resorption of the roots on mesial surfaces of teeth subjected to a large orthodontic force (50 g). Therefore, the present model represents a method for inducing efficient tooth movement and root resorption. Furthermore, Hayashi et al.9 established a method for achieving a jiggling movement in rats. We referred to this method and produced a force applications in opposing directions force by moving the roots of rat molars bucco-palatally once a week for 21 days in vivo.
In the A area in this study, the H&E results in the OF and HF groups were largely consistent with those of previous studies.10 The application of a jiggling force increased resorption lacunae in comparison with the HF group on day 21 (Figure 4). Conversely, in the B area, many resorption lacunae were observed in the JF group on days 14 and 21 (Figure 4). Chan and Darendeliler6 quantified the extent of root resorption under compression (150 g) and tension (150 g) in human teeth undergoing OTM using volumetry. The volume of root resorption was greater under compression than under tension or under both compression and tension. Furthermore, there was greater root resorption under both compression and tension than under tension alone. This finding supports the present results. Interestingly, the number of resorption lacunae was significantly greater under the condition of a jiggling force of 10 g than under the condition of a continuous unidirectional force of 50 g during tooth movement in rats (Figure 5). Therefore, weaker jiggling forces may induce more root resorption than stronger continuous forces. Conversely, Eross et al.15 reported no significant difference in root resorption between a heavy continuous force (225 g) and heavy jiggling force (225 g every 4 weeks) in humans. The discrepancies between previous results and the present results may be due to differences between species and the magnitudes and intervals of the applied forces. Further studies are needed to investigate the contribution of jiggling forces to ORR.
ORR occurs at the periphery of necrotic hyalinized tissue,16 and the pattern at the site of compression is related to the lesion.17 The pathogenesis of ORR is associated with the removal of necrotic tissue from areas of the PDLs compressed by orthodontic loads.1819 Previous studies have also shown that ORR is caused by the removal of necrotic hyalinized tissue.2021 Tartrate-resistant acid phosphatase (TRAP) staining in a rat tooth movement model highlighted the involvement of TRAP-positive macrophages and multinucleated giant cells in the removal of hyalinized tissue.18 Recently, Ohashi et al.22 reported that immunoreactivity for TRAP was stronger in resorbed roots exposed to a jiggling force (10 g) versus a heavy force (50 g) on day 21. Therefore, the number of TRAP-positive odontoclasts was significantly increased in the jiggling force group compared with the HF group on day 21.
Previous studies have reported that 2 to 4 weeks are required to remove hyalinized tissue.23 A jiggling movement leads to compression on both the buccal and lingual sides; therefore, the formation of hyalinized tissue may extend over a wide area. Hence, the application of forces in opposing directions before periodontal tissue repair induces the formation of hyalinized tissue, consequently aggravating ORR. Eross et al.15 concluded that jiggling forces applied alternately in different directions with a short interval of reactivation are critically important in inducing severe root resorption.
Cathepsin K-positive and MMP-9-positive odontoclasts were also increased in the A area in the JF group on day 21 compared with the HF and OF groups, and many cathepsin K-positive and MMP-9-positive odontoclasts were observed in the JF group on days 14 and 21 in the B area (Figures 5,6). Osteoclasts and odontoclasts resorb mineralized tissues by lowering the pH in resorption lacunae, followed by tissue degradation via the secretion of proteolytic enzymes, which are classified as either cysteine proteinases, including the cathepsin family, or MMPs. In particular, cathepsin K and MMP-9 are characteristic proteinases expressed in osteoclasts and odontoclasts.2425 Tsuchiya et al.26 reported that cathepsin K and MMP-9 are expressed in odontoclasts under root resorption conditions during tooth movement in rats. Taken together, these findings and the present results suggest that jiggling forces may induce odontoclast formation during OTM.
The relationship between ORR and inflammatory cytokines has been reported in many studies using rat tooth movement models. Heavy forces of 50 g induce ORR via RANKL/OPG, IL-6, and IL-8 production.272829 These findings support the results observed in the OF and HF groups in this study. In our study, at a jiggling force of 10 g (the optimal force), IL-6-positive, CINC-1-positive, and RANKL-positive PDL cells in the A areas were increased in the JF group on day 21 compared with the HF group (50 g) and the OF group (10 g). Many IL-6-positive, CINC-1-positive, and RANKL-positive odontoclasts were also observed in the JF group on days 14 and 21 in the B areas (Figures 7,8,9). In all groups, few OPG-positive cells were observed in PDL tissues during days 7 through 21 (Figure 10).
Considering the mechanism of enhancement of these cytokines by jiggling forces, in vitro studies applying compression and tension forces to PDL cells may provide clues. Previous studies have reported that compression forces induce the production of inflammatory cytokines, such as IL-6, IL-8, and RANKL in a magnitude-dependent manner in human PDL cells in vitro.272829 Interestingly, tension forces also induce these cytokines in PDL cells. Therefore, jiggling forces may increase cytokines more significantly in response to both compression and tension forces than in response to unidirectional forces.
Regarding differences between the A and B areas, the numbers of IL-6-positive, CINC-1-positive, and RANKL-positive cells were greater in the JF group in A than in the JF group in B on day 21 (Figure 11). The PDL tissues in A were exposed to compression forces twice and tension forces once, whereas those in B were exposed to tension forces twice and compression forces once. Garlet et al.30 demonstrated increased expression of RANKL on both the compression and tension sides versus the controls, with significantly greater expression on the compression side than on the tension side. Therefore, a jiggling force may increase cytokines more significantly in the A area than the B area.
Figures and Tables
Figure 1
Experimental tooth movement. Experimental tooth movement was induced by the design of an appliance (diameter: 0.012 inches [0.3048 mm], stainless steel wire) ligated to a maxillary first molar cleat by a 0.008-inches (0.2032 mm) stainless steel ligature wire. The upper first molar was moved palatally or buccally using the appliance with a force of 10 g or 50 g. The appliances were attached to the rats after activation in each direction.
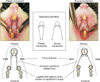
Figure 2
The experimental schedule for each group. The rats were randomly assigned to four groups: the control group with no appliance (days 0–21); the optimal force (OF) group, treated with 10 g of compression (days 0–21); the heavy force (HF) group, treated with 50 g of compression (days 0–21); and the jiggling force (JF) group, treated with 10 g compression on day 7 (days 0–7), 10 g of tension on day 14 (days 8–14), and 10 g of compression (the A area) on day 21 (days 15–21); and 10 g of tension on day 7 (days 0–7), 10 g of compression on day 14 (days 8–14), and 10 g of tension (the B area) on day 21 (days 15–21).

Figure 3
A schematic illustration showing the area of investigation (box) on the palatal aspect of the distal palatal root of the first molar (hematoxylin and eosin staining).
MP, Mesial palatal root; M, mesial root; MB, mesial buccal root; DP, distal palatal root; DB, distal buccal root; M1, the left first upper molar; PDL, periodontal ligament.

Figure 4
Light microscopy images showing the effects of different orthodontic forces on multinucleated osteoclasts (hematoxylin and eosin staining, ×400). The expression of odontoclasts (arrowheads) on the cementum in the jiggling force group (u) was greater than those in the heavy force (o) and optimal force groups (i) on day 21. The direction of the applied force is indicated by the large arrow.
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 5
Effects of different orthodontic forces on the expression of cathepsin K-positive odontoclasts (×400).
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 6
Effects of different orthodontic forces on the expression of matrix metalloproteinase (MMP)-9-positive odontoclasts (×400).
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 7
Effects of different orthodontic forces on the expression of interleukin (IL)-6-positive odontoclasts (×400).
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 8
Effects of different orthodontic forces on the expression of cytokine-induced neutrophil chemoattractant 1 (CINC-1)-positive odontoclasts (×400).
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 9
Effects of different orthodontic forces on the expression of receptor activator of nuclear factor κB ligand (RANKL)-positive odontoclasts (×400).
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 10
Effects of different orthodontic forces on the expression of osteoprotegerin (OPG)-positive odontoclasts (×400).
PDL, Periodontal ligament; C, cementum; D, dentin.

Figure 11
A statistical diagram depicting the quantitative assessment of cellular changes.
MMP, Matrix metalloproteinase; IL, interleukin; CINC-1, cytokine-induced neutrophil chemoattractant 1; RANKL, receptor activator of nuclear factor κB ligand; OPG, osteoprotegerin; Control, control group; OF, optimal force group; HF, heavy force group; JF, jiggling force group.
*p < 0.05, **p < 0.01, significantly different from the corresponding control group; †p < 0.05, ††p < 0.01, significantly different from the corresponding HF.

Notes
References
1. Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991; 61:125–132.
2. Malmgren O, Goldson L, Hill C, Orwin A, Petrini L, Lundberg M. Root resorption after orthodontic treatment of traumatized teeth. Am J Orthod. 1982; 82:487–491.

3. Linge BO, Linge L. Apical root resorption in upper anterior teeth. Eur J Orthod. 1983; 5:173–183.

4. Owman-Moll P, Kurol J, Lundgren D. The effects of a four-fold increased orthodontic force magnitude on tooth movement and root resorptions. An intraindividual study in adolescents. Eur J Orthod. 1996; 18:287–294.

5. Owman-Moll P, Kurol J, Lundgren D. Effects of a doubled orthodontic force magnitude on tooth movement and root resorptions. An inter-individual study in adolescents. Eur J Orthod. 1996; 18:141–150.

6. Chan E, Darendeliler MA. Physical properties of root cementum: Part 5. Volumetric analysis of root resorption craters after application of light and heavy orthodontic forces. Am J Orthod Dentofacial Orthop. 2005; 127:186–195.

7. Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 2. Literature review. Am J Orthod Dentofacial Orthop. 1993; 103:138–146.

8. Mirabella AD, Artun J. Risk factors for apical root resorption of maxillary anterior teeth in adult orthodontic patients. Am J Orthod Dentofacial Orthop. 1995; 108:48–55.

9. Hayashi A, Hayashi H, Kawata T. Prevention of root resorption in hypofunctional teeth by occlusal function recovery. Angle Orthod. 2015; DOI: 10.2319/012215-47.1. [Epub ahead of print].

10. Nakano Y, Yamaguchi M, Fujita S, Asano M, Saito K, Kasai K. Expressions of RANKL/RANK and M-CSF/c-fms in root resorption lacunae in rat molar by heavy orthodontic force. Eur J Orthod. 2011; 33:335–343.

11. Kaku M, Sumi H, Shikata H, Kojima S, Motokawa M, Fujita T, et al. Effects of pulpectomy on the amount of root resorption during orthodontic tooth movement. J Endod. 2014; 40:372–378.

12. Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008; 78:502–509.

13. Kohno T, Matsumoto Y, Kanno Z, Warita H, Soma K. Experimental tooth movement under light orthodontic forces: rates of tooth movement and changes of the periodontium. J Orthod. 2002; 29:129–135.

14. Gameiro GH, Nouer DF, Pereira-Neto JS, de Araújo Magnani MB, de Andrade ED, Novaes PD, et al. Histological analysis of orthodontic root resorption in rats treated with the cyclooxygenase-2 (COX-2) inhibitor celecoxib. Orthod Craniofac Res. 2008; 11:156–161.

15. Eross E, Turk T, Elekdag-Turk S, Cakmak F, Jones AS, Végh A, et al. Physical properties of root cementum: Part 25. Extent of root resorption after the application of light and heavy buccopalatal jiggling forces for 12 weeks: A microcomputed tomography study. Am J Orthod Dentofacial Orthop. 2015; 147:738–746.

16. Brudvik P, Rygh P. Non-clast cells start orthodontic root resorption in the periphery of hyalinized zones. Eur J Orthod. 1993; 15:467–480.

17. Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 1. Literature review. Am J Orthod Dentofacial Orthop. 1993; 103:62–66.

18. Brudvik P, Rygh P. Root resorption beneath the main hyalinized zone. Eur J Orthod. 1994; 16:249–263.

19. Kurol J, Owman-Moll P. Hyalinization and root resorption during early orthodontic tooth movement in adolescents. Angle Orthod. 1998; 68:161–165.
20. Kvam E. Scanning electron microscopy of tissue changes on the pressure surface of human premolars following tooth movement. Scand J Dent Res. 1972; 80:357–368.

21. Rygh P. Ultrastructural cellular reactions in pressure zones of rat molar periodontium incident to orthodontic tooth movement. Acta Odontol Scand. 1972; 30:575–593.

22. Ohashi M, Yamaguchi M, Hikida T, Kikuta J, Shimizu M, Goseki T, et al. Jiggling force induces orthodontic root resorption during tooth movement in rats. Int Oral-Med Sci. 2015; 14:13–20.

23. Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006; 28:221–240.

24. Wucherpfennig AL, Li YP, Stetler-Stevenson WG, Rosenberg AE, Stashenko P. Expression of 92 kD type IV collagenase/gelatinase B in human osteoclasts. J Bone Miner Res. 1994; 9:549–556.

25. Oshiro T, Shibasaki Y, Martin TJ, Sasaki T. Immunolocalization of vacuolar-type H+-ATPase, cathepsin K, matrix metalloproteinase-9, and receptor activator of NFkappaB ligand in odontoclasts during physiological root resorption of human deciduous teeth. Anat Rec. 2001; 264:305–311.

26. Tsuchiya M, Akiba Y, Takahashi I, Sasano Y, Kashiwazaki J, Tsuchiya S, et al. Comparison of expression patterns of cathepsin K and MMP-9 in odontoclasts and osteoclasts in physiological root resorption in the rat molar. Arch Histol Cytol. 2008; 71:89–100.

27. Asano M, Yamaguchi M, Nakajima R, Fujita S, Utsunomiya T, Yamamoto H, et al. IL-8 and MCP-1 induced by excessive orthodontic force mediates odontoclastogenesis in periodontal tissues. Oral Dis. 2011; 17:489–498.

28. Hayashi N, Yamaguchi M, Nakajima R, Utsunomiya T, Yamamoto H, Kasai K. T-helper 17 cells mediate the osteo/odontoclastogenesis induced by excessive orthodontic forces. Oral Dis. 2012; 18:375–388.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download