Abstract
The incorporation of technological advances in the field of clinical orthodontics to increase treatment efficiency has led to the development of customized appliances (Insignia®), archwires (Suresmile®), and the production of devices to enhance tooth movement (Acceledent®). This review presents a comprehensive study of the literature concerning these products, and analyzes the available evidence of their efficiency. To date, one pilot study has evaluated the efficiency of the Insignia® system, three retrospective studies have assessed the efficiency of the Suresmile® system, and a few Acceledent® reports have described its effect on treatment time. Critical appraisal of the reviewed papers revealed that the efficiency of the Insignia® system cannot be confirmed based on the available evidence, while the use of Suresmile® can reduce overall treatment time in simple cases. The acceleration of tooth movement by Acceledent® devices has not yet been confirmed.
Developments in three-dimensional imaging and manufacturing processes have made the customization of orthodontic appliances to improve treatment efficiency possible. Advances in technology have yielded two patient-specific products that utilize computers to create an interactive treatment plan, and then manufacture a custom-designed appliance: the Insignia® system (Ormco Corporation, Orange, CA, USA) and Suresmile® archwires (OraMetrix, Inc., Richardson, TX, USA). The potential benefits of accelerating tooth movement to expedite orthodontic treatment have also driven the marketing of the Acceledent® vibrational devices (OrthoAccel Technologies, Inc., Bellaire, TX, USA) (Figure 1). This report presents a comprehensive review of the literature relating to these products, and analyzes the available evidence of their efficiency.
The Insignia® system provides clinicians with software that helps them virtually design the final occlusion, and then brackets and archwires are reverse-engineered to move teeth to the desired outcome. The company offers patient-specific brackets, indirect-bonding transfer jigs, and custom archwires.12 In 2009, an Insignia® user Dr. David Sarver recommended the Insignia® system due to its "... ability to design treatment as individually as possible, rather than a '1 size fits all' approach. It allows us to truly plan treatment with the end in mind".3 Gracco et al.4 have recently published a clinical report on the main features and the clinical advantages of the Insignia® system. They demonstrated the treatment efficiency of the system while treating a 16-year-old male who presented with a Class II malocclusion for 17 months without the need to rebond the brackets or bend the archwires.
The only report available to date on the efficiency of the Insignia® system was presented as a pilot study by Weber et al.2 The records of 35 cases treated with the Insignia® system were compared to 11 cases treated conventionally, in terms of the quality of the results and treatment times. The American Board of Orthodontics (ABO) scores were lower in the Insignia® group, indicating that the finished results were closer to those defined by the ideal ABO criteria. In addition, the mean treatment time was significantly shorter in cases treated with Insignia® (14.23 months vs. 22.91 months), and those patients were treated via approximately seven fewer appointments on average than the conventionally treated patients. In their discussion of the results, the authors acknowledge that the sample size of the conventionally treated group was small and that the initial peer assessment rating scores were low in both groups. Therefore, the findings of this study may not apply to patients with more severe malocclusions.2 Randomized clinical trials with larger sample sizes are required, to assess the effectiveness and efficiency of the Insignia® custom bracket system.2
Suresmile® provides customized archwires that help clinicians in the finishing stage. The process of fabricating these archwires involves digital imaging with intraoral scanners or cone-beam computed tomography, a review of the final position of the teeth using Suresmile® software, and the production of prescription archwires by robots.
The Chief Clinical Officer at OraMetrix, Inc., Dr. Sachdeva, first described the clinical procedure of the Suresmile® system in 2001.56 An updated protocol appeared later, with the modification that teeth were scanned 3−5 months after the initial alignment.7 That paper included a report of seven Suresmile® cases, and a statistical comparison of the overall treatment times between cases treated with the Suresmile® system and conventionally treated patients. The mean treatment times reported were 12.1 months and 23.1 months respectively; however, no selection criteria were described and the data collection methodology was not reported.7
In a published review, Moles8 has reported that the average treatment time of 500 cases treated with Suresmile® was 13.1 months. Dr. Nicole M. Jane,9 an advisor for Suresmile®, reported that the average treatment time using Suresmile® ranged from 14 to 6 months, while for conventionally treated cases it ranged from 20 to 22 months.
To date, only three retrospective studies have evaluated the quality of the finished results produced by Suresmile®, and its efficiency. Saxe et al.10 compared the treatment times of 38 Suresmile® cases with those of 24 traditionally treated cases from three orthodontic practices. That study revealed that Suresmile® patients were treated faster than the conventionally treated patients, and their results were also of better quality. The discrepancy in the pre-treatment severity of malocclusion between the two groups was not statistically analyzed, although it was different. Several critical issues relating to the design of that study have been noted, including the lack of randomization, the small sample size, and the fact that the authors multiplied data and reported measurements of 76 cases instead of the 38 actual Suresmile® patients.11
Alford et al.12 compared the treatment times of 69 Suresmile® cases with those of 63 conventionally treated cases from one orthodontic practice. They reported that Suresmile® was associated with a shorter treatment time, and resulted in a lower ABO cast-radiograph evaluation score. However, the initial malocclusions of the Suresmile® patients were less severe than those of the conventionally treated cases.
In a recently published study, Sachdeva et al.13 compared the treatment times of 9,390 patients treated with Suresmile® with those of 2,945 patients treated conventionally, and the difference was statistically significant at p < 0.001. The authors declared that no standardization or calibration measures were applied during data collection. Since no defined selection criteria for the conventionally treated cases were reported, it is possible that the majority of these cases were treated with extraction, which would definitely affect overall treatment times.11
Acceledent® devices have been marketed with the aim of enhancing tooth movement during orthodontic treatment.14 They have a mouthpiece that applies cyclic vibrational forces directly to the teeth. Patients are instructed to use the device and activate it once daily for 20 minutes. The company website refers to Dr. Mao's animal studies151617 on the effect of the application of cyclic forces on the acceleration of bone remodeling processes as the foundation for scientific research in the field of enhancing tooth movement by vibrating forces.18 Nishimura et al.19 reported an animal study which showed that 8 minutes of resonance vibrational activity applied once a week for 3 weeks led to a 15% (approximately 0.18 mm) increase in the rate of tooth movement.
Kau et al.20 reported the results of an uncontrolled clinical trial of 14 patients who completed a 6-month study period during which they wore the Acceledent® device for 20 minutes daily. The authors used the change in the displacement of teeth (Little's irregularity index)21 as a direct measure of the rate of tooth movement, without reporting any calibration method, and that was 0.526 mm per week or 2.1 mm per 28 days, in the mandibular arch.20
A retrospective, non-randomized study with two similar control groups on the efficiency of Acceledent® was reported by Bowman22 in 2014. The author reported that alignment in the Acceledent® group was 27 days faster on average (p = 0.0988, not statistically significant), and levelling was 48 days faster in the Acceledent® group (p = 0.0486, significant) compared to the study control. The achievement of alignment and levelling was based on the subjective decision of the clinician and was not calibrated.22
Woodhouse et al.23 have recently published the results of a prospective, randomized clinical trial with a non-functional (sham) device control group, and fixed appliances only control group, that were compared to an Acceledent® group. The study limited the investigation to the rate of tooth alignment and not the complete duration of treatment, and it found that the use of vibrational forces does not significantly reduce the amount of time required to achieve tooth alignment.23
OrthoAccel Technologies, Inc. has funded a clinical trial of which the lead author also serves as a consultant to the company.24 Pavlin et al.24 reported allocating 45 patients aged 12−40 years to be treated with Acceledent® device or a sham appliance (control). Rate of canine retraction was measured directly in the patient's mouth, but the measurement error was not reported.25 The average rate (mm/month) of tooth movement in the Acceledent® group was 1.16, while in the control group was 0.79, and the difference 0.37 was statistically significant (p = 0.05, 95% CI –0.07 to 0.81). However, the confidence interval of the difference between the means included zero and this suggests no significant difference.25
The efficiency of orthodontic treatment facilitated by Acceledent® devices has not been demonstrated in a reliable, independent, randomized, controlled trial. There is no study on the effect of Acceledent® devices on total treatment time, and the underlying mechanism of action of these vibrational forces has not been clarified.26 The safety or otherwise of these devices, and the possible side effects associated with them, have also not been documented in any studies as yet.26
The available evidence does not support a valid conclusion of the efficiency of the Insignia® system. While retrospective studies suggest a possible reduction in the total treatment time with the use of Suresmile® for simple malocclusion cases mainly. Independent studies have not shown acceleration of tooth movement with Acceledent® yet and the action mechanism, side effects, and complications were not reported.
A summary of the analysis of the studies reviewed with regard to the efficiency of Insignia®, Suresmile®, and Acceledent® is shown in Table 1.
References
1. Ormco-Corporation. Increasing clinical performance with 3D interactive treatment planning and patient-specific appliances. Orange, CA: Ormco-Corporation;2006.
2. Weber DJ 2nd, Koroluk LD, Phillips C, Nguyen T, Proffit WR. Clinical effectiveness and efficiency of customized vs. conventional preadjusted bracket systems. J Clin Orthod. 2013; 47:261–266. quiz 268. PMID: 23660822.
3. Scholz RP, Sarver DM. Interview with an Insignia doctor: David M. Sarver. Am J Orthod Dentofacial Orthop. 2009; 136:853–856. PMID: 19962609.

4. Gracco A, Stellini E, Parenti SI, Bonetti GA. Individualized orthodontic treatment: The Insignia system. Orthodontics (Chic.). 2013; 14:e88–e94. PMID: 23646343.

5. Mah J, Sachdeva R. Computer-assisted orthodontic treatment: the SureSmile process. Am J Orthod Dentofacial Orthop. 2001; 120:85–87. PMID: 11455383.

6. Sachdeva RC. SureSmile technology in a patient--centered orthodontic practice. J Clin Orthod. 2001; 35:245–253. PMID: 11345571.
7. Sachdeva R, Frugé JF, Frugé AM, Ingraham R, Petty WD, Bielik KL, et al. SureSmile: a report of clinical findings. J Clin Orthod. 2005; 39:297–314. quiz 315. PMID: 15961890.
8. Moles R. The SureSmile system in orthodontic practice. J Clin Orthod. 2009; 43:161–174. quiz 184. PMID: 19458453.
9. Jane NM. Interview with a SureSmile doctor: Nicole M. Jane. Interview by Robert P. Scholz. Am J Orthod Dentofacial Orthop. 2009; 135(4 Suppl):S140–S143. PMID: 19362265.
10. Saxe AK, Louie LJ, Mah J. Efficiency and effectiveness of SureSmile. World J Orthod. 2010; 11:16–22. PMID: 20209172.
11. Alford TJ. SureSmile, an unbiased review (Lecture). Paper presented at: 113th Annual Session of the American Association of Orthodontists. 2013 May 4-7; Philadelphia, PA. Philadelphia: American Association of Orthodontists;2013.
12. Alford TJ, Roberts WE, Hartsfield JK Jr, Eckert GJ, Snyder RJ. Clinical outcomes for patients finished with the SureSmile™ method compared with conventional fixed orthodontic therapy. Angle Orthod. 2011; 81:383–388. PMID: 21261488.

13. Sachdeva RC, Aranha SL, Egan ME, Gross HT, Sachdeva NS, Currier GF, et al. Treatment time: SureSmile vs conventional. Orthodontics (Chic.). 2012; 13:72–85. PMID: 22567618.
14. Kau CH. A novel device in orthodontics. Aesthet Dent Today. 2009; 3:42–43.
15. Kopher RA, Mao JJ. Suture growth modulated by the oscillatory component of micromechanical strain. J Bone Miner Res. 2003; 18:521–528. PMID: 12619937.

16. Peptan AI, Lopez A, Kopher RA, Mao JJ. Responses of intramembranous bone and sutures upon in vivo cyclic tensile and compressive loading. Bone. 2008; 42:432–438. PMID: 18032124.

17. Vij K, Mao JJ. Geometry and cell density of rat craniofacial sutures during early postnatal development and upon in vivo cyclic loading. Bone. 2006; 38:722–730. PMID: 16413234.

18. OrthoAccel® Technologies Inc. Clinical evidence [Internet]. Houston, TX: OrthoAccel® Technologies Inc.;2014. cited 2014 Sep 9. Available from: http://acceledent.com/orthodontists/clinical-evidence.
19. Nishimura M, Chiba M, Ohashi T, Sato M, Shimizu Y, Igarashi K, et al. Periodontal tissue activation by vibration: intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2008; 133:572–583. PMID: 18405822.

20. Kau CH, Nguyen JT, English JD. The clinical evaluation of a novel cyclical force generating device in orthodontics. Orthod Pract US. 2010; 1:10–15.
21. Little RM. The irregularity index: a quantitative score of mandibular anterior alignment. Am J Orthod. 1975; 68:554–563. PMID: 1059332.

22. Bowman SJ. The effect of vibration on the rate of leveling and alignment. J Clin Orthod. 2014; 48:678–688. PMID: 25707947.
23. Woodhouse NR, DiBiase AT, Johnson N, Slipper C, Grant J, Alsaleh M, et al. Supplemental vibrational force during orthodontic alignment: a randomized trial. J Dent Res. 2015; 94:682–689. PMID: 25758457.
24. Pavlin D, Anthony R, Raj V, Gakunga PT. Cyclic loading (vibration) accelerates tooth movement in orthodontic patients: a double-blind, randomized controlled trial. Semin Orthod. 2015; 21:187–194.

25. AcceleDent again. Cyclic vibration accelerates tooth movement; A clinical trial! [Internet]. Kevin O'Brien's Orthodontic Blog;2015. 6. 22. cited 2016 Jan 16. Available from: http://kevinobrienorthoblog.com/acceledent-again-cyclic-vibration-acceleratestooth-movement-a-clinical-trial.
26. Proffit WR. Accelerated tooth movement? Surgery first? Third molar management? (Lecture). Paper presented at: 114th Annual Session of the American Association of Orthodontists. 2014 Apr 25-29; New Orleans, LA. New Orleans: American Association of Orthodontists;2014.
Figure 1
The orthodontic appliances reviewed. A, An image derived from the Insignia® system software (Ormco Corporation, Orange, CA, USA). B, An image derived from the Suresmile® software (OraMetrix, Inc., Richardson, TX, USA). C, The Acceledent® Aura device (OrthoAccel Technologies, Inc., Bellaire, TX, USA).
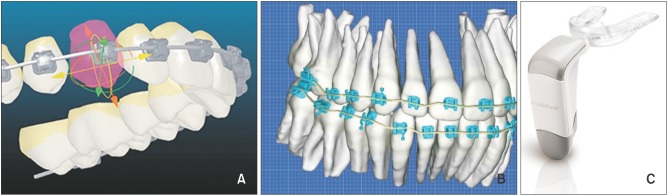
Table 1
A summary of the studies reviewed relating to the efficiency of Insignia®, Suresmile®, and Acceledent® systems
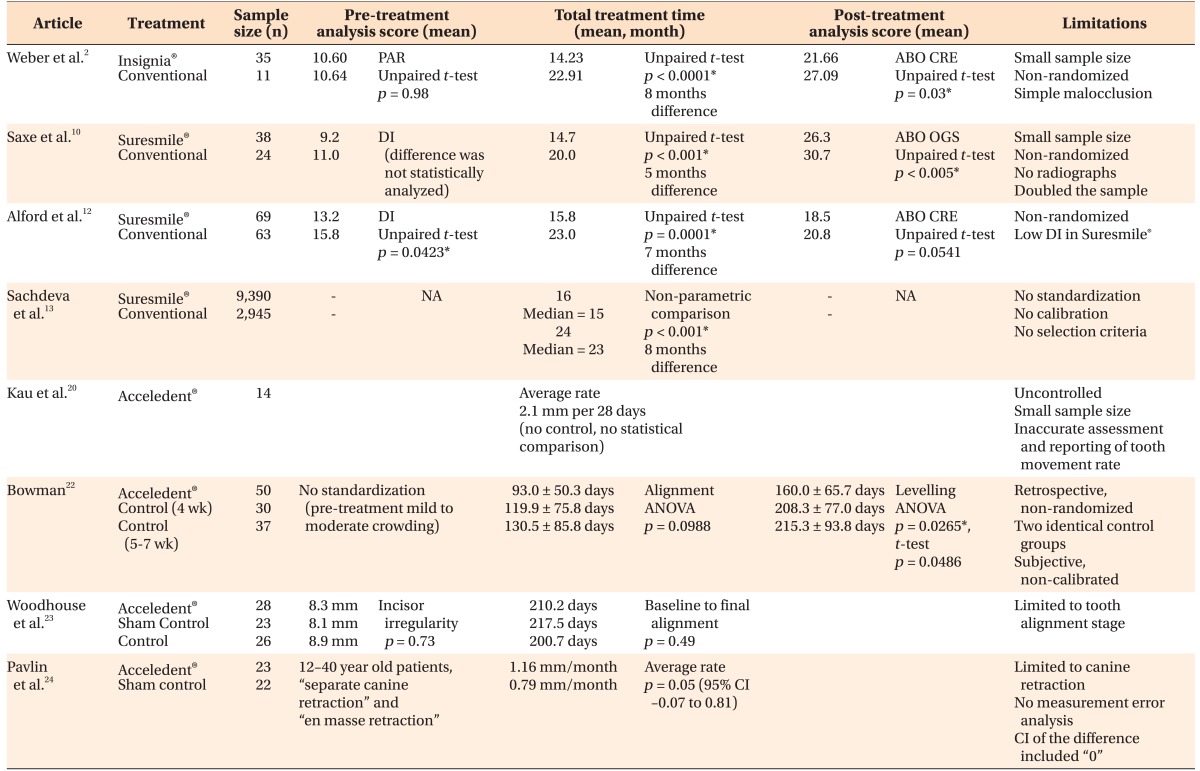
Insignia®, Ormco Corporation, Orange, CA, USA; Suresmile®, OraMetrix, Inc., Richardson, TX, USA; Acceledent®; OrthoAccel Technologies, Inc., Bellaire, TX, USA.
PAR, Peer assessment rating index; DI, discrepancy index; ABO, American Board of Orthodontics; OGS, objective grading system; CRE, cast-radiograph evaluation; NA, not available; CI, confidence interval.
*Statistically significant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download