Abstract
Condylar hyperplasia (CH) is a rare disorder characterized by excessive bone growth that almost always presents unilaterally, resulting in facial asymmetry. Classification of the different types of CH can differ depending on the authors. Correct diagnosis is critical in determining the proper treatments and timing. This paper is a review of the recent literature on the epidemiology, etiology, diagnosis, classification, and surgical treatments of CH.
Condylar hyperplasia (CH) is a rare disorder characterized by excessive bone growth that usually presents unilaterally, resulting in facial asymmetry.1 Its etiology and pathogenesis are poorly understood. Facial asymmetry is often the reason that patients present for treatment of the disorder. In many cases, occlusal discrepancies and temporomandibular joint disorder are concurrent symptoms with facial asymmetry. An attempt has been made to separate and classify different types of CH based on clinical characteristics and etiology.2 In 1986, Obwegeser and Makek3 specifically detailed two hemimandibular anomalies, hemimandibular hyperplasia and hemimandibular elongation. These anomalies can be clinically present in a pure form or in combination. Many diagnostic tools and criteria have been used to aid in the correct diagnosis of CH, which in turn is critical to determining the appropriate treatments and timing. With proper diagnosis, timing, and treatment, CH can be effectively treated with a high success rates.
CH can occur at any age and can continue past the growth period.4 Previous literature has suggested that CH may predominantly affect women.4 A meta-analysis was conducted by Raijmakers et al.5 in 2012 to assess this hypothesis, which revealed that women develop CH significantly more frequently, with 64% (95% CI, 58-70%; n = 275 patients) of CH patients being women. CH was also proposed to demonstrate sex-based laterality, with women and men affected more frequently on the right and left side, respectively.4 However, the meta-analysis by Raijmakers et al.5 did not find any evidence that the side affected by CH was linked to sex. Current research has yet to define an exact etiology for CH. Possible etiologies include endocrine distortions (e.g., insulin-like growth factors [IGFs]), metabolic hyperactivity, trauma, arthrosis, and genetics. Typical mandibular condyle soft tissue histology includes four layers: fibrous articular layer, undifferentiated mesenchymal layer, transitional layer, and hypertrophic cartilage layer.6 Active CH has been found to display a broader mesenchymal layer than that in the normal condyle.7
IGF-1 and IGF-1 receptor (IGF-1R) expression was found to significantly increase in chondrocytes affected by CH.7 IGF-1 is an essential growth modulator that has been described as a pathological factor when produced in excess8 and 'has been shown to reach higher-than-normal levels in CH'.79 In an in vitro model, Chen et al.7 demonstrated that IGF-1 was able to significantly increase the proliferation of chondrocytes from the cartilage of normal condyles and, conversely, that NVP-AEW541 (an inhibitor of IGF-1R kinase) was able to significantly decrease the proliferation capacity of chondrocytes cultured from CH. Gene expression of collagen type II A1 (COL2A1) was found to significantly increase in CH compared to that in normal condylar cartilage, and when chondrocytes from normal condylar cartilage were treated with IGF-1, gene expression of COL2A1 also significantly increased. However, when CH chondrocytes were treated with NVP-AEW541, no significant reduction in COL2A1 expression occurred.7 These results indicate that IGF-1 has an impact on chondrogenesis of the mandibular condyle; however, the extent and full underlying mechanism of this effect is not known.
A study by Saridin et al.10 analyzed metabolism and blood flow in seven patients (mean age 25.5 years) with unilateral CH using 18F and H215O positron emission tomography (PET) imaging. Four patients had hemimandibular elongation, one had hemimandibular hyperplasia, and two had a combination of these anomalies. Their results highlighted the possibility that CH can also be caused by a decrease in growth activity of the contralateral condyle with normal growth in the affected condyle. In patients with condylar hyperactivity, 18F uptake, used to assess bone formation, was significantly higher in the affected condyle than in the contralateral condyle.10 However, the 18F uptake of the hyperactive condyles was similar to that of control condyles, while the contralateral condyles had significantly less 18F uptake than control condyles.10 These results suggest that in this patient sample, the affected condyle grows at a normal rate, while a cessation of growth occurs in the contralateral condyle. In contrast to previous literature,11 the study by Saridin et al.10 did not find any relationship between bone formation and blood flow.
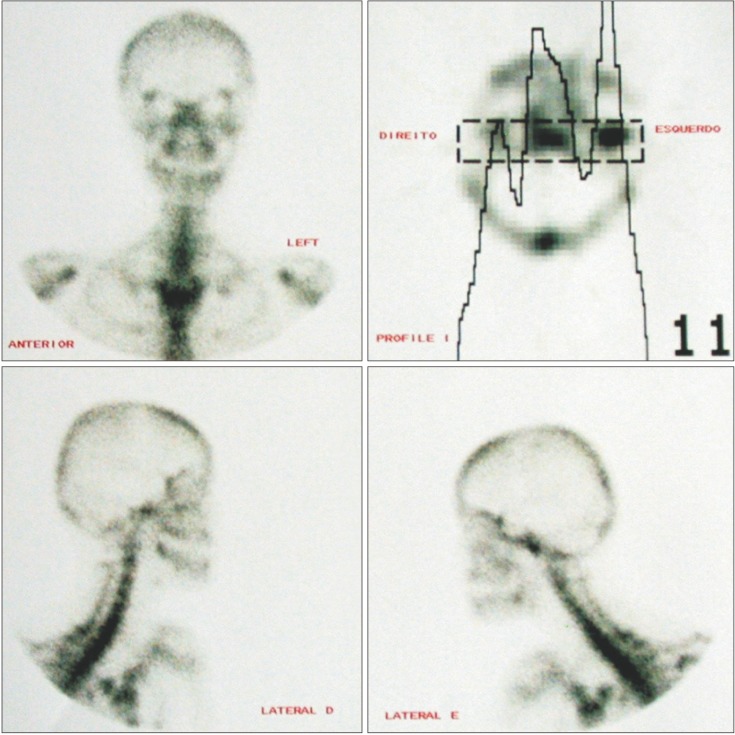
Various methods are available for the diagnosis of CH. Correct diagnosis of CH is essential when deciding how to treat the condition. To prevent post-surgical reversion, accurate diagnosis of CH activity is also of upmost importance.12 Diagnostic methods such as clinical examination, radiographs, and nuclear imaging (Figure 1) can be used to determine the type of CH as well as its activity. Clinical diagnosis has been described as the diagnostic gold standard.13
Nuclear imaging is capable of providing physiological details of CH using radionuclide-labeled tracers.14 Examples of different types of nuclear imaging include planar scintigraphy, single-photon emission computed tomography (SPECT) and PET. SPECT and planar scintigraphy utilize the radionucleotide technetium-99m-labelled methylene diphosphonate (99mTc-MDP), while PET utilizes the radionucleotide [18F]-fluoride.15 Prior to the development of 99mTc-MDP, [18F]-fluoride was the standard radionucleotide tracer for SPECT.15 Planar scintigraphy produces a two-dimensional image, as opposed to SPECT and PET, which produce three-dimensional images. Bone scintigraphy has high sensitivity and low specificity for bone metabolism, meaning that it can identify when a change in bone metabolism is present but is limited in its ability to differentiate among various conditions (e.g., bone healing, growth, infection, arthritic changes, or tumors).14
Generally when condyles are being evaluated with bone scintigraphy, a difference in uptake levels of less than 10% indicates either normal condyles or individuals without progressive asymmetry.13 A meta-analysis by Saridin et al.13 found that the SPECT technique of bone scintigraphy had a significantly higher sensitivity (0.90) in detecting unilateral CH than the planar technique (0.71) (p = 0.04). However, no difference in specificity was found between these two techniques. PET has been described as having better spatial resolution than SPECT.15 Further research is needed to establish a more formalized method for scintigraphy analysis. The current literature provides various methods such as comparing right and left condylar activity in the form of a percentage or ratio and comparing condylar activity to a different bony landmark such as the lumbar vertebrae.13 An attempt was made to relate SPECT findings to histopathological differences in CH, in which SPECT was found insufficiently sensitive to detect histopathological differences.16
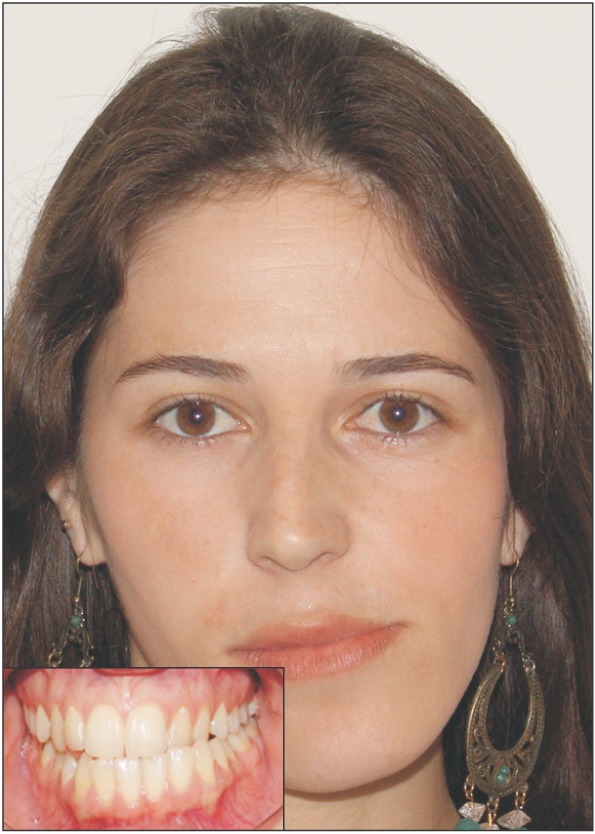
Temporomandibular joint CH has been described as a rare unilateral growth of the mandibular condyle. This growth causes both functional and esthetic problems, which often manifest as facial asymmetry, occlusal interferences (Figure 2), and joint dysfunction that can lead to pain.1 Excessive growth can occur in several different locations in the mandible. The growth can be the result of an enlarged condyle, an elongated condylar neck, or outward bowing or downward growth of the body and ramus.17 Due to the variations in locations of excessive growth, multiple classification systems have been developed to better characterize the pathology.
Obwegeser and Makek3 developed a classification system based on the asymmetry and predominant growth vector (Table 1). In their article, they classified CH into 3 different categories. They defined Type 1 as hemimandibular elongation, with excessive growth displayed in the horizontal vector. Type 1 CH is associated with chin deviation toward the unaffected side, with no corresponding vertical asymmetry. Due to the overgrowth, the mandibular midline is also shifted to the contralateral side. As a result, the contralateral mandibular molars often deviate lingually in order to remain in proper occlusion with the maxillary molars. If the contralateral molars are unable to adapt to the growth, a crossbite may develop. Normally, the condyle is unaffected, but the neck is often misshapen and slender. The ramus is elongated, which is the basis for referring to Type 1 as hemimandibular elongation. Type 2 CH was defined as hemimandibular hyperplasia, which is associated with excessive growth in the vertical vector. It is often characterized by a sloping rima oris with minimal chin deviation. Due to the excessive downward growth of the mandible, the maxillary molars on the affected side compensate by following the mandible's downward growth. The maxillary alveolar bone on the ipsilateral side grows excessively to maintain occlusion. If the maxillary molars are not able to follow the excessive downward growth, an open bite on the affected side results. In Type 2 CH, the condyle often appears enlarged, and the head is usually irregular or deformed. The neck of the condyle has also been reported as thickened and/or elongated.18 Type 3 CH is a combination of Types 1 and 2.
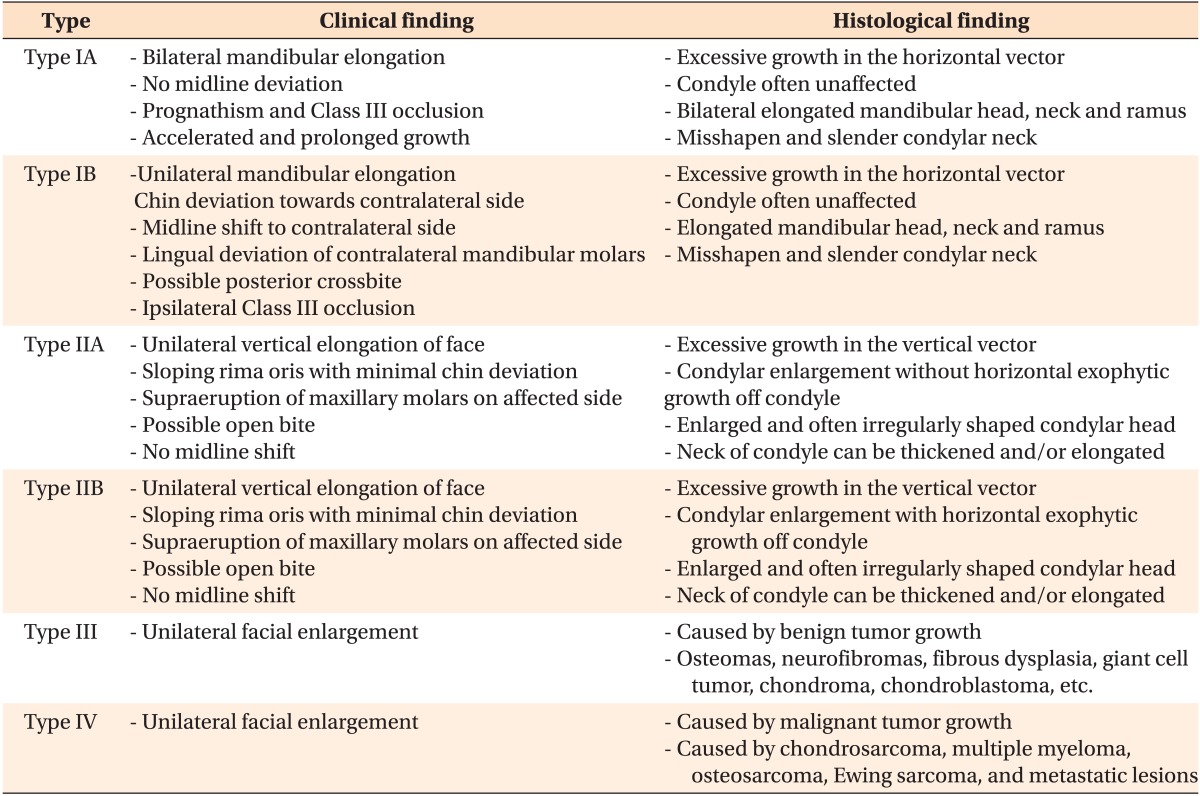
Wolford et al.2 developed an updated classification system that they considered more inclusive of pathologies causing CH. Their report classifies CH into four different categories based on clinical, imaging, growth, and histological characteristics. This system was developed to classify CH into more specific types in order to provide optimal treatment to patients based on their specific disease characteristics (Table 2).
In this system, Type 1 and 2 CH are similar to the classification system developed by Obwegeser and Makek3 with the following exceptions. Type 1 is characterized by an accelerated and prolonged growth that causes condylar and mandibular elongation and split into 1A and 1B. CH Type 1A is defined as mandibular elongation that occurs bilaterally, while CH Type 1B occurs unilaterally. CH Type 2 consists of unilateral overgrowth of the condyle caused by an osteochondroma and results in vertical overgrowth of the mandible. Wolford et al.19 further classified CH Type 2A and B. Type 2A results from vertical elongation of the condylar head and neck. Type 2B involves horizontal exophytic tumor growth of the condyle in addition to vertical elongation of the head and neck. CH Type 3 consists of other benign tumors that cause CH, including but not limited to osteomas, neurofibromas, and fibrous dysplasia, and results in unilateral facial enlargement. Type 4 CH is caused by malignant tumors that originate in the condyle and cause enlargement and facial asymmetry. Some malignant tumors attributed to Type 4 CH include chondrosarcoma, multiple myeloma, osteosarcoma, and Ewing sarcoma.
Classification systems have also been created based on histological findings in CH patients. Slootweg and Müller20 were among the first to create a histological classification system based on a study they conducted in 1986, in which they classified 22 patients into four categories based on histological findings in various layers of hyperplastic condyles. Specifically, they analyzed the fibrous articular layer, the undifferentiated mesenchymal layer, the transitional layer, and the hypertrophic cartilage layer and characterized each layer based on histological findings. CH Type 1 displayed a broad proliferation zone with an underlying thick layer of hyaline growth cartilage and bone that contained numerous cartilage islands. CH Type 2 demonstrated a patchier distribution of proliferation zones with a smaller number of cartilage islands. CH Type 3 was characterized by irregular-shaped masses of cartilage found in the bone of the condylar neck or encroaching onto the superficial articular layer. Type 3 displayed great distortion compared to the histological findings of normal condyles. Type 4 CH was commonly characterized by a burned-out appearance of the condyle due to a very cell-poor fibrocartilaginous layer covering the subchondral bone plate. Slootweg and Müller20 also noted that Type 4 CH did not demonstrate a proliferation layer of the hyaline growth cartilage like that seen in the other types.
One of the first steps in managing CH cases is to determine if the mandible is actively growing. This determination can be made with many methods, but bone SPECT is an essential diagnostic tool to assess active growth.21 In this quantitative method, 99mTc-MDP is injected and absorbed into hydroxyapatite crystals and calcium in the bone.22 The bone is then scanned using the SPECT technique, and the hyperplastic condyle is quantitatively compared to the contralateral side.23 Often only a 0-5% difference in positive area is observed between normal condyles. Differences greater than 10% between two condyles are considered to indicate active growth due to CH.12 Therefore, a relative 55% uptake in the affected hyperplastic condyle is considered to be abnormal. Various other diagnostic methods can be used in addition to clinical examinations. Some other tests used to diagnose CH include three-dimensional tomography, standard radiology and cephalometry, and PET scans.24
Once a detailed diagnosis of CH has been obtained, a treatment plan must be established. Treatment is primarily surgical and often accompanied by orthodontics to correct occlusion. There is some controversy as to the ideal treatment option and time to treat. Treatment plans must consider the degree of asymmetry, resulting malocclusion, and condylar growth activity. Treatments to correct these problems can be approached jointly or separately. Usually, the selected strategy is dependent on growth activity and the patient's age. As always, the patient's demands and expectations are other important considerations. A study by Naini et al.25 found that the patient's desire for surgical intervention increases as the degree of asymmetry increases. Interestingly, they also found that asymmetries as small as 5 mm can be noticed by the laypersons.
CH treatment options are detailed from the simplest, least invasive to most complex procedures. One of the simplest procedures that can be performed is a mandibular ramus osteotomy of affected condyles. Motamedi26 treated 13 CH patients over a 10-year period with ramus osteotomies. Seven of the patients were treated with bilateral osteotomies alone, six were treated with unilateral osteotomies, and two of those procedures were combined with Le Fort I procedures. The study concluded that patients with unilateral CH can effectively be treated with unilateral ramus osteotomies on the affected side. Bilateral osteotomies did not show any advantages over unilateral procedures; however, they may be indicated for patients with severely prognathic profiles and patients in whom unilateral osteotomies could possibly lead to excessive rotation of the unaffected condyle. Combining the osteotomies with Le Fort I is effective in restoring occlusal discrepancies.
Many studies and case reports advocate high condylectomies to treat CH. An analysis of 22 patients treated with high condylectomies who completed a 4-year follow-up concluded that this method is a viable treatment option for patients, which often has a good and predictable surgical outcome.20 Lippold et al.27 also found that high condylectomy was an appropriate treatment for unilateral CH. In their study, 4-5 mm was removed from the upper pole of the affected condyle, which appeared to effectively limit growth in CH.
The most complex surgical treatment for CH involves both a condylectomy and orthognathic surgery. In a classic study conducted by Wolford et al.19 in 2002, a group of patients was treated with both a high condylectomy and orthognathic surgery, and this treatment was found to be very effective for correcting both functional and esthetic problems resulting from CH. Another study supporting this claim found that combined condylectomy and orthognathic surgery was successful both functionally and esthetically in 30 of 36 patients.28 However, it also indicated that orthognathic surgery without any condylar intervention could possibly lead to future issues, because condylar growth may not be complete at the time of surgery. Thus, without treating the hyperplastic condyle, growth could possibly continue following orthognathic surgery.
By contrast, orthognathic surgery alone may be an acceptable treatment if the excessive condylar growth has halted. Two studies treated a combined 44 CH patients after the excessive condylar growth was determined to have ceased.29 The cessation of condylar growth was confirmed by taking multiple SPECT images over a determined time period to compare the amount of active growth in each condyle. For all surgical options, timing and the patient's wishes are critical in determining the correct treatment plan for CH.
The literature pertaining to orthodontic treatment in patients with CH is very limited and generally consists of case reports. In a report of five cases by Rajkumar et al.30, the authors' final assessment was that mild to severe CH can be treated with surgery alone. Other case reports have described the use of orthodontic treatment after surgical treatment to finalize occlusion. In a case involving two-stage surgery, Xavier et al.31 described their procedure as consisting of condylectomy followed by orthodontic treatment and orthognathic surgery. Each of these reports only describes the treatment used and what worked in those particular circumstances. No comparative literature is available on which to base a reliable treatment recommendation. If orthodontic treatment is chosen, it is vital to confirm that growth has halted before the orthodontic treatment begins.
CH requires a thorough examination and diagnostic tools to accurately diagnose and treat. Patients often seek treatment due to facial asymmetry and resulting esthetic problems. Additional research is needed to establish a more standardized approach for diagnosing the activity of CH. Clinical examination, radiography, planar scintigraphy, SPECT, and PET are all diagnostic methods that can be utilized by clinicians when planning surgery. Longer follow-up studies must be completed to determine which treatment options are the most successful. Additionally, more studies are needed to understand the underlying causes of CH for earlier diagnosis and better treatment options.
References
2. Wolford LM, Movahed R, Perez DE. A classification system for conditions causing condylar hyperplasia. J Oral Maxillofac Surg. 2014; 72:567–595. PMID: 24388179.

3. Obwegeser HL, Makek MS. Hemimandibular hyperplasia--hemimandibular elongation. J Maxillofac Surg. 1986; 14:183–208. PMID: 3461097.

4. Nitzan DW, Katsnelson A, Bermanis I, Brin I, Casap N. The clinical characteristics of condylar hyperplasia: experience with 61 patients. J Oral Maxillofac Surg. 2008; 66:312–318. PMID: 18201615.

5. Raijmakers PG, Karssemakers LH, Tuinzing DB. Female predominance and effect of gender on unilateral condylar hyperplasia: a review and meta-analysis. J Oral Maxillofac Surg. 2012; 70:e72–e76. PMID: 21856058.

6. Hansson T, Oberg T, Carlsson GE, Kopp S. Thickness of the soft tissue layers and the articular disk in the temporomandibular joint. Acta Odontol Scand. 1977; 35:77–83. PMID: 266827.

7. Chen Y, Ke J, Long X, Meng Q, Deng M, Fang W, et al. Insulin-like growth factor-1 boosts the developing process of condylar hyperplasia by stimulating chondrocytes proliferation. Osteoarthritis Cartilage. 2012; 20:279–287. PMID: 22281262.

8. Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004; 363:1346–1353. PMID: 15110491.

9. Götz W, Lehmann TS, Appel TR, Rath-Deschner B, Dettmeyer R, Luder HU, et al. Distribution of insulin-like growth factors in condylar hyperplasia. Ann Anat. 2007; 189:347–349. PMID: 17695990.

10. Saridin CP, Raijmakers PG, Kloet RW, Tuinzing DB, Becking AG, Lammertsma AA. No signs of metabolic hyperactivity in patients with unilateral condylar hyperactivity: an in vivo positron emission tomography study. J Oral Maxillofac Surg. 2009; 67:576–581. PMID: 19231783.

11. Li QF, Rabie AB. A new approach to control condylar growth by regulating angiogenesis. Arch Oral Biol. 2007; 52:1009–1017. PMID: 17640614.

12. Pripatnanont P, Vittayakittipong P, Markmanee U, Thongmak S, Yipintsoi T. The use of SPECT to evaluate growth cessation of the mandible in unilateral condylar hyperplasia. Int J Oral Maxillofac Surg. 2005; 34:364–368. PMID: 16053843.

13. Saridin CP, Raijmakers PG, Tuinzing DB, Becking AG. Bone scintigraphy as a diagnostic method in unilateral hyperactivity of the mandibular condyles: a review and meta-analysis of the literature. Int J Oral Maxillofac Surg. 2011; 40:11–17. PMID: 20970961.

14. Lewis EL, Dolwick MF, Abramowicz S, Reeder SL. Contemporary imaging of the temporomandibular joint. Dent Clin North Am. 2008; 52:875–890. PMID: 18805233.

15. Laverick S, Bounds G, Wong WL. [18F]-fluoride positron emission tomography for imaging condylar hyperplasia. Br J Oral Maxillofac Surg. 2009; 47:196–199. PMID: 18926607.

16. Fariña RA, Becar M, Plaza C, Espinoza I, Franco ME. Correlation between single photon emission computed tomography, AgNOR count, and histomorphologic features in patients with active mandibular condylar hyperplasia. J Oral Maxillofac Surg. 2011; 69:356–361. PMID: 21122972.

17. Gray RJ, Sloan P, Quayle AA, Carter DH. Histopathological and scintigraphic features of condylar hyperplasia. Int J Oral Maxillofac Surg. 1990; 19:65–71. PMID: 2111360.

18. Bruce RA, Hayward JR. Condylar hyperplasia and mandibular asymmetry: a review. J Oral Surg. 1968; 26:281–290. PMID: 4867494.
19. Wolford LM, Mehra P, Reiche-Fischel O, Morales-Ryan CA, García-Morales P. Efficacy of high condylectomy for management of condylar hyperplasia. Am J Orthod Dentofacial Orthop. 2002; 121:136–150. PMID: 11840126.

20. Slootweg PJ, Müller H. Condylar hyperplasia. A clinico-pathological analysis of 22 cases. J Maxillofac Surg. 1986; 14:209–214. PMID: 3461098.

21. Alyamani A, Abuzinada S. Management of patients with condylar hyperplasia: A diverse experience with 18 patients. Ann Maxillofac Surg. 2012; 2:17–23. PMID: 23483790.

22. Olate S, Netto HD, Rodriguez-Chessa J, Alister JP, de Albergaria-Barbosa J, de Moraes M. Mandible condylar hyperplasia: a review of diagnosis and treatment protocol. Int J Clin Exp Med. 2013; 6:727–737. PMID: 24179565.
23. Saridin CP, Gilijamse M, Kuik DJ, te Veldhuis EC, Tuinzing DB, Lobbezoo F, et al. Evaluation of temporomandibular function after high partial condylectomy because of unilateral condylar hyperactivity. J Oral Maxillofac Surg. 2010; 68:1094–1099. PMID: 20149509.

24. Mehrotra D, Dhasmana S, Kamboj M, Gambhir G. Condylar hyperplasia and facial asymmetry: report of five cases. J Maxillofac Oral Surg. 2011; 10:50–56. PMID: 22379321.

25. Naini FB, Donaldson AN, McDonald F, Cobourne MT. Assessing the influence of asymmeftry affecting the mandible and chin point on perceived attractiveness in the orthognathic patient, clinician, and layperson. J Oral Maxillofac Surg. 2012; 70:192–206. PMID: 21571417.

26. Motamedi MH. Treatment of condylar hyperplasia of the mandible using unilateral ramus osteotomies. J Oral Maxillofac Surg. 1996; 54:1161–1169. PMID: 8859233.

27. Lippold C, Kruse-Losler B, Danesh G, Joos U, Meyer U. Treatment of hemimandibular hyperplasia: the biological basis of condylectomy. Br J Oral Maxillofac Surg. 2007; 45:353–360. PMID: 17145124.

28. Villanueva-Alcojol L, Monje F, González-García R. Hyperplasia of the mandibular condyle: clinical, histopathologic, and treatment considerations in a series of 36 patients. J Oral Maxillofac Surg. 2011; 69:447–455. PMID: 20828911.

29. Yamashita Y, Nakamura Y, Shimada T, Nomura Y, Hirashita A. Asymmetry of the lips of orthognathic surgery patients. Am J Orthod Dentofacial Orthop. 2009; 136:559–563. PMID: 19815159.

30. Rajkumar GC, Muralidoss H, Ramaiah S. Conservative management of unilateral condylar hyperplasia. Oral Maxillofac Surg. 2012; 16:201–205. PMID: 22200752.

31. Xavier SP, Santos Tde S, Silva ER, Faria AC, de Mello Filho FV. Two-stage treatment of facial asymmetry caused by unilateral condylar hyperplasia. Braz Dent J. 2014; 25:257–260. PMID: 25252264.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download