Abstract
Many clinicians expect complete healing after the removal of temporary anchorage devices, but clinical examination may reveal scar-like tissue. This report presents the typical features of scarring detected after the removal of miniscrews, and the clinical outcome of scar revision along with its pathologic features.
Favored implantation sites for orthodontic miniscrews are the interdental alveolar bone within the attached gingiva and the palate. These sites are considered anatomically safe, provide sufficient stability, and are covered by non-movable soft tissue.1,2,3,4 These sites are reportedly safe zones with regard to scar formation,5,6 and as miniscrews are of limited size, many clinicians assume complete healing after miniscrew removal. However, distinguishable scar-like tissue is frequently detected after miniscrew removal.7
Scar-like tissue at the miniscrew removal site is characterized by specific morphological features, coloration, and texture. In general, the scar-like tissue is localized to the removal site with a small, elevated, lump-like morphology matching the size of the miniscrew diameter, is whitish in color, distinguishable from the adjacent reddish-pink gingiva or the oral mucosa, and exhibits a firm texture upon palpation as compared to the adjacent tissue (Figure 1).7 Small scars do not cause functional disturbances, and in many cases are not detected by the patient or the practitioner, but esthetic and informed consent issues may arise when visible scarring is evident after treatment.
To our knowledge, neither the pathology of scarring after miniscrew removal nor options to overcome these soft tissue limitations has been documented in the orthodontic literature. The objectives of this report were to illustrate the typical features of soft tissue scars detected after the removal of miniscrews, and to present a clinical case of scar revision.
A female patient in her twenties came to the clinic with the chief complaints of crowding and a gummy smile. The patient showed mild crowding with excessive gingival display and relatively short clinical crown height (Figure 2A). Molar and canine occlusion showed a Class I relationship.
The patient desired simple anterior alignment without changes to her molar occlusion. Therefore, the treatment objectives were set to improve anterior alignment and incisor display only. Esthetic mini-tubes and two-dimensional lingual brackets were bonded to the six anterior teeth of the maxilla and the mandible respectively. To improve the anterior dental esthetics, gingivectomy was planned with mild intrusion of the maxillary anterior teeth. Two self-drilling orthodontic miniscrews (1016107; Ortholution, Seoul, Korea) were inserted distal to the maxillary canines at the mucogingival junction, to apply intrusive force similar to that of J-hook headgear (Figure 2B).8 After 4 months, appliances were removed following gingivectomy (Figure 2C).
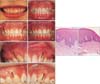
After 3 years, the occlusion itself was stably maintained. However, small whitish lumps were observed at the miniscrew sites. The lesions were distinguished from the adjacent tissue by a protuberant appearance and whitish coloration (Figure 2D, 2E, and 2F at greater magnification). There was no associated discomfort, or pain on palpation, but it was visually distinct from the adjacent gingiva and the mucosa. The lesions were exposed at full smile, and the patient felt self-conscious and desired removal of the lesions.
The lesions were clinically diagnosed as scars remaining after the removal of miniscrews.7 The scar tissues were excised as a form of scar revision under local anesthesia. After 3 months, the sites became flat (Figure 2G and 2H). Histologic evaluation of excisional biopsy samples of the scar and adjacent tissue confirmed hyperkeratosis, and dense collagen fibers of the dermis with flat and smoother rete pegs compared to the adjacent gingival tissue (Figure 2I and 2J).
The removal of miniscrews is generally not considered a traumatic procedure. However, it leaves a transitory full thickness defect penetrating the oral cavity, soft tissue, and underlying alveolar bone, which is healed by secondary intention. Scar formation is considered a common result of the healing process, with dense accumulation of collagen bundles and reduced extracellular matrix turnover of fibroblasts.5,9 Because wound regeneration including maturation and remodeling usually continues for up to 12 months,10,11 scars detected after a year are expected to remain, as in the present case, rather than disappear with time.
On clinical examination, scar lesions were localized to the removal site, and presented as small lumps with distinguishing coloration paler than that of the adjacent tissue. Hyperkeratosis may explain the whitish color, and the dense collagen matrix in the dermal layer is considered responsible for the external shape.12 Taken together, the clinical and histological features reported herein suggest that the scar lesions are similar to those of a "hypertrophic scar" of the skin. Hypertrophic scarring is characterized by excessive deposition of extracellular matrix proteins limited to the boundary of the original wound. It occurs when the inflammatory response is prolonged with increased vascularization, hypercellularity, and excessive collagen deposition.9,13 Patient-related factors such as age, skin type, and genetics; wound-related factors such as site of trauma, size, and type of inflammation; and environmental factors such as mechanical loading play major roles in hypertrophic scar formation.14,15
It has been reported that the overall prevalence of visible scarring after miniscrew removal was higher than expected, at approximately 44.6%, and intrinsic host factors such as flat gingival biotype and miniscrew insertion site in the maxillary buccal interdental regions were associated with increased susceptibility to scarring.7 It has also been suggested that the removal of failed miniscrews may hinder healing due to persistent infection.16 In the patient reported herein, the miniscrews were stable without any sign of inflammation during their usage, and the insertion time was relatively short. However, the patient belonged to the high-risk group with a flat gingival biotype, defined as relatively short and wide crown form of the upper central incisors with wide attached gingiva,17 and the insertion site at the buccal interdental region.
Surgical excision followed by histological evaluation for differential diagnosis was selected as the treatment of choice for scar revision, because no other non-invasive procedures were available. Although the patient was fully satisfied with the clinical outcome, subjects who are susceptible to scarring may display repeated intra-oral scar formation after surgical removal. Currently, a lack of published scientific studies hinders the prediction of scarring and the development of preventative measures. Additional miniscrew studies should focus on methods to improve soft tissue healing after their temporary application, and reducing irreversible scar formation.
Visible soft tissue scarring can develop after the use of temporary anchorage devices. Although such scarring is limited to the site of insertion and small in size, it may cause esthetic issues. Surgical removal is possible, but further studies on methods to improve soft tissue healing and to prevent visible scarring are necessary.
Figures and Tables
Figure 1
Typical soft tissue scars after miniscrew removal. The scar tissue was localized to the removal sites and exhibited a protuberant appearance with clear margins and whitish coloration (arrows). A and D, Female in 20s, distal to upper canine. Immediately after (A) and 34 months after miniscrew removal (D). B and E, Female in 20s, upper molar region. Immediately after (B) and 18 months after miniscrew removal (E). C and F, Male in 20s, palatal slope. Immediately after (C) and 10 months after (F) the removal of the miniscrew. The arrows indicate the site of miniscrew removal.
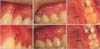
Figure 2
Serial intra-oral photographs and histological evaluation of the scar tissue. A, Initial presentation; B, during orthodontic treatment; C, after miniscrew removal; D, 36 months after miniscrew removal; E and F, before scar revision (right and left, respectively); G and H, 3 months after scar revision (right and left, respectively). Hematoxylin-Eosin staining of the excised scar tissue; I, adjacent normal gingiva; J, scar tissue showing hyperkeratosis and dense extracellular fibers in the dermis. The arrows indicate the site of miniscrew removal.
Notes
References
1. Lee KJ, Joo E, Kim KD, Lee JS, Park YC, Yu HS. Computed tomographic analysis of tooth-bearing alveolar bone for orthodontic miniscrew placement. Am J Orthod Dentofacial Orthop. 2009; 135:486–494.

2. Sawada K, Nakahara K, Matsunaga S, Abe S, Ide Y. Evaluation of cortical bone thickness and root proximity at maxillary interradicular sites for mini-implant placement. Clin Oral Implants Res. 2013; 24:Suppl A100. 1–7.

3. Schätzle M, Männchen R, Zwahlen M, Lang NP. Survival and failure rates of orthodontic temporary anchorage devices: a systematic review. Clin Oral Implants Res. 2009; 20:1351–1359.

4. Choi JH, Yu HS, Lee KJ, Park YC. Three-dimensional evaluation of maxillary anterior alveolar bone for optimal placement of miniscrew implants. Korean J Orthod. 2014; 44:54–61.

5. Larjava H, Wiebe C, Gallant-Behm C, Hart DA, Heino J, Häkkinen L. Exploring scarless healing of oral soft tissues. J Can Dent Assoc. 2011; 77:b18.
6. Mak K, Manji A, Gallant-Behm C, Wiebe C, Hart DA, Larjava H, et al. Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. J Dermatol Sci. 2009; 56:168–180.

7. Jung SA, Choi YJ, Lee DW, Kim KH, Chung CJ. Cross-sectional evaluation of the prevalence and factors associated with soft tissue scarring after the removal of miniscrews. Angle Orthod. 2014; [Epub ahead of print].

8. Lim JK. Gummy smile correction using mini implant. Korean J Clin Orthod. 2010; 9:14–31.
9. Zhu Z, Ding J, Shankowsky HA, Tredget EE. The molecular mechanism of hypertrophic scar. J Cell Commun Signal. 2013; 7:239–252.

10. Bond JS, Duncan JA, Sattar A, Boanas A, Mason T, O'Kane S, et al. Maturation of the human scar: an observational study. Plast Reconstr Surg. 2008; 121:1650–1658.

11. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008; 453:314–321.

12. Pietrokovski J, Massler M. Ridge remodeling after tooth extraction in rats. J Dent Res. 1967; 46:222–231.

13. Mahdavian Delavary B, van der Veer WM, Ferreira JA, Niessen FB. Formation of hypertrophic scars: evolution and susceptibility. J Plast Surg Hand Surg. 2012; 46:95–101.

14. Wolfram D, Tzankov A, Pülzl P, Piza-Katzer H. Hypertrophic scars and keloids--a review of their pathophysiology, risk factors, and therapeutic management. Dermatol Surg. 2009; 35:171–181.

15. Kim JH, Sung JY, Kim YH, Lee YS, Chang HS, Park CS, et al. Risk factors for hypertrophic surgical scar development after thyroidectomy. Wound Repair Regen. 2012; 20:304–310.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download