Abstract
Objective
To three-dimensionally elucidate the effects of occlusal hypofunction on the periodontal ligament and alveolar bone proper of rat molars by micro-computed tomography (micro-CT).
Methods
Occlusal function in the molar area was restricted by attaching an anterior bite plate on the maxillary incisors and a metal cap on the mandibular incisors of 5-week-old male Wistar rats for 1 week. The periodontal ligament space and alveolar bone proper around roots of the mandibular first molar were assessed by histology and micro-CT.
Results
The periodontal ligament space was narrower and the alveolar bone proper was sparser and less continuous in the hypofunction group than in the control group. Further, both the volume of the periodontal ligament and the volumetric ratio of the alveolar bone proper to the total tissue in the region of interest were significantly lower in the hypofunction group (p < 0.05).
The compact bone lining the tooth socket, where periodontal ligament (PDL) fibers attach, is known as bundle bone or alveolar bone proper. It is easily identifiable in radiographs and histological sections. The PDL and alveolar bone proper have a close relationship: the PDL provides progenitor cells for bone formation and remodeling.1,2,3,4
Occlusal hypofunction leads to atrophic changes in the periodontium.5,6,7,8,9,10,11,12 These changes include narrowing of the PDL space, vascular constriction, and deformation of the mechanoreceptor structure (Ruffini endings). Therefore, occlusal stimuli are essential for maintaining the structural integrity of the periodontium.5,6,7,11,12
Previous histological studies have shown regressive changes in the periodontium of rat molars after loss of occlusal stimuli,7,11,12 but three-dimensional morphometric analysis has not yet been reported. In this study, we aimed to three-dimensionally elucidate the effects of occlusal hypofunction on the PDL and alveolar bone proper of rat molars by micro-computed tomography (micro-CT).
Five-week-old male Wistar rats (n = 18) were randomly divided into two equal groups. The control group was not treated. To restrict occlusal function in the hypofunction group, an anterior bite plate was attached on the maxillary incisors and a metal cap constructed from orthodontic band material (4.6 × 0.13 mm; Rocky Mountain Morita Corp., Tokyo, Japan) was attached on the mandibular incisors by using photopolymerizing composite resin (Clearfil Liner Bond 2; Kuraray Dental, Okayama, Japan) (Figure 1A).8,9,10 Body weight was monitored daily.8,9,10 One week later, the animals were sacrificed by cervical dislocation under diethyl ether anesthesia (Wako Pure Chemical Ind. Ltd., Osaka, Japan).8,10 The experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Tokyo Medical and Dental University (approval number #0130279A).
The mandibles were dissected and most soft tissue was removed. The specimens were immediately immersed in 10% buffered formalin (Wako Pure Chemical Ind. Ltd.; pH 7.0) at 4℃ overnight and decalcified in 10% ethylenediaminetetraacetic acid (EDTA) at 4℃ for 6 weeks. They were embedded in paraffin and cut into 5-µm-thick sagittal sections parallel to the long axis of the roots of the mandibular first molar.8,10 The sections were finally mounted on glass slides coated with poly-L-lysine (Matsunami Glass Ind. Ltd., Osaka, Japan) and stained with hematoxylin and eosin for histomorphometric examination. The observational area included the dental pulp, PDL, alveolar bone proper, and inter-radicular alveolar bone (Figure 1B).
The histological observational area was imaged by using a desktop X-ray micro-CT system (SMX-90CT; Shimadzu Corp., Kyoto, Japan) with a scanning resolution of 20 µm. First, the mesial and distal roots of the mandibular first molar were manually located at 20 µm intervals on individual images. The selected images were compiled into a three-dimensional image by using image-analysis software (TRI/3-D Bon; Ratoc System Engineering Co. Ltd., Tokyo, Japan). The region of interest was enlarged to a 40 µm radius around the roots.8,9,10 The software was then used for data quantification. Tissue volume (TV) was defined as the volume of tissue in the enlarged region of interest. The bone-to-tissue volume (BV/TV) ratio was calculated as the volumetric ratio of bone to total tissue in the enlarged region of interest. Both parameters were evaluated according to the software instructions.
No significant differences in body weight were noted between the groups at the time of killing.
Histologically, the PDL space in the hypofunction group was narrower than that in the control group (Figure 1C). Furthermore, the alveolar bone proper showed less continuity in the hypofunction group (Figure 1C).
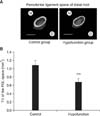
Figure 2A shows that the PDL space around the mesial root was thinner in the hypofunction group than in the control group. Identical narrowing of the PDL space was observed around the distal root in the hypofunction group (Figure 3A). As shown in Figure 4A, the alveolar bone proper around the mesial root was more discontinuous in the hypofunction group. Furthermore, the alveolar bone proper around the distal root was sparser in this group (Figure 5A).
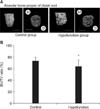
In response to occlusal hypofunction, the PDL volume around both the roots significantly reduced (Figures 2B and 3B). The BV/TV ratio of the alveolar bone proper was also significantly lower around both the roots in the hypofunction group (Figures 4B and 5B).
We reduced occlusal function in the molar area by using an anterior bite plate on the maxillary incisors and a metal cap on the mandibular incisors.8,9,10 We selected the mandibular first molar as the region of interest because it is exposed to concentrated occlusal stimuli and has been used in previous studies of alveolar bone.8,10 By micro-CT, we succeeded in building a three-dimensional representation of the PDL space and alveolar bone proper.
Occlusal hypofunction caused the molar PDL space to become significantly narrow, consistent with previous reports5,6,7,8,9,10,11,12 and supporting the view that occlusal stimuli are important for thickening PDL fibers and maintaining the PDL space.6,7,11,12 Further, the alveolar bone proper was discontinuous and its BV/TV ratio was significantly reduced. We determined the adaptive threshold of bone by using the "Discriminant Analysis" function of the image-analysis software. Bone images were also visually examined with more than the calculated threshold values to confirm the accuracy of our estimations. This adaptive threshold was then used to discriminate areas of bone from areas of other tissue and was kept constant across all the examined sections.
Mechanical stimuli are important for maintaining bone.13 Alveolar bone is particularly responsive because of its high turnover under mechanical stress (e.g., occlusal force) transmitted from the teeth through the PDL.14,15 When occlusal force is absent, osteoclast numbers are boosted by the expression of the osteoclast differentiation factor, receptor activator of nuclear factor kappa-B ligand (RANKL), which leads to bone resorption.8,10 Cellular components of the PDL participate in the formation and resorption of periodontal hard tissues.16,17,18 Therefore, discontinuity of the alveolar bone proper in this hypofunction model may have risen from suppression of bone formation and/or acceleration of bone resorption by cellular components of the PDL. Further investigation is needed to reveal how osteoblasts and osteoclasts in the PDL affect the alveolar bone proper under conditions of occlusal hypofunction.
Figures and Tables
Figure 1
A, The experimental model; B, histological observational area (rectangular area); C, representative hematoxylin and eosin-stained sections (Scale = 250 µm). M, Mesial; D, distal; PL, periodontal ligament; AP, alveolar bone proper; AB, inter-radicular alveolar bone.
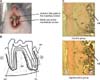
Figure 2
A, Three-dimensional reconstructed images of the periodontal ligament (PDL) space (Scale = 1 mm); B, comparison of tissue volume (TV) around the mesial root of the mandibular first molar.
***p < 0.001 by the Mann-Whitney U-test.
M, Mesial; D, distal.
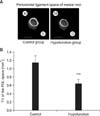
Figure 3
A, Three-dimensional reconstructed images of the periodontal ligament (PDL) space (Scale = 1 mm); B, comparison of tissue volume (TV) around the distal root of the mandibular first molar.
***p < 0.001 by the Mann-Whitney U-test.
M, Mesial; D, distal.
Notes
References
1. Gault P, Black A, Romette JL, Fuente F, Schroeder K, Thillou F, et al. Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol. 2010; 37:750–758.

2. Sebaoun JD, Kantarci A, Turner JW, Carvalho RS, Van Dyke TE, Ferguson DJ. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008; 79:1679–1688.

3. Devlin H, Sloan P. Early bone healing events in the human extraction socket. Int J Oral Maxillofac Surg. 2002; 31:641–645.

4. Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007; 10:149–160.

5. Hayashi Y, Iida J, Warita H, Soma K. Effects of occlusal hypofunction on the microvasculature and endothelin expression in the periodontal ligaments of rat molars. Orthod Waves. 2001; 60:373–380.
6. Tanaka A, Iida J, Soma K. Effect of hypofunction on the microvasculature in the periodontal ligament of the rat molar. Orthodontic Waves. 1998; 57:180–188.
7. Muramoto T, Takano Y, Soma K. Time-related changes in periodontal mechanoreceptors in rat molars after the loss of occlusal stimuli. Arch Histol Cytol. 2000; 63:369–380.

8. Enokida M, Kaneko S, Yanagishita M, Soma K. Inference of occlusal stimuli on the remodelling of alveolar bone in a rat hypofunction-recovery model. J Oral Biosci. 2005; 47:321–334.

9. Shimomoto Y, Chung CJ, Iwasaki-Hayashi Y, Muramoto T, Soma K. Effects of occlusal stimuli on alveolar/jaw bone formation. J Dent Res. 2007; 86:47–51.

10. Shimizu Y, Hosomichi J, Kaneko S, Shibutani N, Ono T. Effect of sympathetic nervous activity on alveolar bone loss induced by occlusal hypofunction in rats. Arch Oral Biol. 2011; 56:1404–1411.

11. Motokawa M, Terao A, Karadeniz EI, Kaku M, Kawata T, Matsuda Y, et al. Effects of long-term occlusal hypofunction and its recovery on the morphogenesis of molar roots and the periodontium in rats. Angle Orthod. 2013; 83:597–604.

12. Watarai H, Warita H, Soma K. Effect of nitric oxide on the recovery of the hypofunctional periodontal ligament. J Dent Res. 2004; 83:338–342.

13. Bourrin S, Palle S, Genty C, Alexandre C. Physical exercise during remobilization restores a normal bone trabecular network after tail suspension-induced osteopenia in young rats. J Bone Miner Res. 1995; 10:820–828.

14. Vignery A, Baron R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat Rec. 1980; 196:191–200.

15. Johnson RB. Effect of altered occlusal function on transseptal ligament and new bone thicknesses in the periodontium of the rat. Am J Anat. 1990; 187:91–97.

16. Meeran NA. Cellular response within the periodontal ligament on application of orthodontic forces. J Indian Soc Periodontol. 2013; 17:16–20.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download