1. Boyd RL, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod. 1992; 62:117–126.
2. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003; 28:215–235.
3. Wood DP, Jordan RE, Way DC, Galil KA. Bonding to porcelain and gold. Am J Orthod. 1986; 89:194–205.

4. Zachrisson BU, Buyukyilmaz T. Recent advances in bonding to gold, amalgam and porcelain. J Clin Orthod. 1993; 27:661–675.
5. Antoniadou M, Kern M, Strub JR. Effect of a new metal primer on the bond strength between a resin cement and two high-noble alloys. J Prosthet Dent. 2000; 84:554–560.

6. Lee YK, Cha JY, Yu HS, Hwang CJ. Effect of metal primer and thermocycling on shear bonding strength between the orthodontic bracket and gold alloy. Korean J Orthod. 2009; 39:320–329.

7. Nergiz I, Schmage P, Herrmann W, Ozcan M. Effect of alloy type and surface conditioning on roughness and bond strength of metal brackets. Am J Orthod Dentofacial Orthop. 2004; 125:42–50.

8. Jung MH, Chung SH, Shon WJ. Shear bond strength between gold alloy and orthodontic metal bracket using light emitting diode curing light. Korean J Orthod. 2010; 40:27–33.

9. Shon WJ, Kim TW, Chung SH, Jung MH. The effects of primer precuring on the shear bond strength between gold alloy surfaces and metal brackets. Eur J Orthod. 2012; 34:72–76.

10. Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984; 85:333–340.

11. Bishara SE, Ajlouni R, Laffoon JF. Effect of thermocycling on the shear bond strength of a cyanoacrylate orthodontic adhesive. Am J Orthod Dentofacial Orthop. 2003; 123:21–24.

12. Büyükyilmaz T, Zachrisson BU. Improved orthodontic bonding to silver amalgam. Part 2. Lathe-cut, admixed, and spherical amalgams with different intermediate resins. Angle Orthod. 1998; 68:337–344.
13. Büyükyilmaz T, Zachrisson YO, Zachrisson BU. Improving orthodontic bonding to gold alloy. Am J Orthod Dentofacial Orthop. 1995; 108:510–518.

14. Bulbul M, Kesim B. The effect of primers on shear bond strength of acrylic resins to different types of metals. J Prosthet Dent. 2010; 103:303–308.

15. Matsumura H, Shimoe S, Nagano K, Atsuta M. Effect of noble metal conditioners on bonding between prosthetic composite material and silver-palladium-copper-gold alloy. J Prosthet Dent. 1999; 81:710–714.

16. Yoshida K, Atsuta M. Effect of MMA-PMMA resin polymerization initiators on the bond strengths of adhesive primers for noble metal. Dent Mater. 1999; 15:332–336.

17. Sun R, Suansuwan N, Kilpatrick N, Swain M. Characterisation of tribochemically assisted bonding of composite resin to porcelain and metal. J Dent. 2000; 28:441–445.

18. Atsü SS, Gelgör IE, Sahin V. Effects of silica coating and silane surface conditioning on the bond strength of metal and ceramic brackets to enamel. Angle Orthod. 2006; 76:857–862.
19. Watanabe T, Ino S, Okada S, Katsumata Y, Hamano N, Hojo S, et al. Influence of simplified silica coating method on the bonding strength of resin cement to dental alloy. Dent Mater J. 2008; 27:16–20.

20. Matinlinna JP, Lassila LV, Ozcan M, Yli-Urpo A, Vallittu PK. An introduction to silanes and their clinical applications in dentistry. Int J Prosthodont. 2004; 17:155–164.
21. Jung MH, Shon WJ, Park YS, Chung SH. Effects of silanation time on shear bond strength between a gold alloy surface and metal bracket. Korean J Orthod. 2013; 43:127–133.

22. Schneider W, Powers JM, Pierpont HP. Bond strength of composites to etched and silica-coated porcelain fusing alloys. Dent Mater. 1992; 8:211–215.

23. Cozza P, Martucci L, De Toffol L, Penco SI. Shear bond strength of metal brackets on enamel. Angle Orthod. 2006; 76:851–856.
24. Patusco VC, Montenegro G, Lenza MA, Alves de Carvalho A. Bond strength of metallic brackets after dental bleaching. Angle Orthod. 2009; 79:122–126.

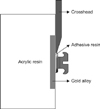








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download