Abstract
Objective
To examine the prophylactic potential of 3 orthodontic bonding adhesives: Fuji Ortho SC, Illuminate, and Resilience.
Methods
Thirty-six Wistar Wag rats were randomly divided into 4 groups consisting of 9 rats each. One of the groups received no treatment and was used as a control. In the other groups, individual bands coated with one of the 3 adhesives were cemented to the lower incisors. Enamel samples were obtained after 6 and 12 weeks and analyzed using scanning electron microscopy in combination with energy dispersive spectrometry.
Results
Six weeks after band cementation, no fluoride was found in the enamel of the lower incisors. After 12 weeks, there was no fluoride in the enamel of teeth coated with the Resilience composite. However, in the case of the Illuminate composite and the resin-modified glass ionomer Fuji Ortho SC cement, the depth of fluoride penetration reached 2 µm and 4.8 - 5.7 µm, respectively.
In contemporary orthodontics, the importance of prophylaxis is increasingly being recognized. In what it concerns to dental caries, early diagnosis and prevention using fluoride are necessary to reduce the need for caries treatment. Nevertheless, multiple options are available for treating caries. Historically, phosphate and polycarboxylic cements, as well as epoxide and acrylic resins, were applied for cementing orthodontic elements on tooth surfaces.1-3 In the 1970s, methacrylic resins were introduced. Nowadays, conventional glass ionomer and resin-modified glass ionomer cements are often used. More recently, fluoride-releasing orthodontic cements have started becoming more popular, for they constitute a constant source of fluorine, independent of the patient's cooperation.4
This development is of special importance for patients with fixed appliances, in whom the frequency of caries can be as high as 96%.5,6 In this group of patients, tooth decay is noted despite regular brushing with fluoride-containing paste, use of interdental brushes, and a low-sugar diet.6 Hence, it appears that perfect hygiene of the oral cavity - despite difficulties such as the limited ability of the tongue to remove food debris - is not sufficient to prevent dental caries in orthodontic patients.
Demineralization of the tooth enamel is primarily attributed to the growth of acid-producing bacteria.7 First, Streptococcus mutans favors the accumulation of dental plaque and the aggregation of bacterial colonies. Lactobacillus acidophilus continues this process, giving rise to the development of secondary caries. To worsen the scenario, placement of a fixed appliance is known to increase the proliferation of S. mutans and L. acidophilus8 and to promote biofilm formation around and between the brackets and in the cervical area of the tooth. Moreover, it is known that demineralization occurs on the surface of teeth with high carbohydrate retention and low saliva flow, e.g., the upper incisors.9 In these teeth, the pH of the dental plaque can be as low as 4.10 On the other hand, demineralization is significantly less frequent on the lingual surface of the lower incisors, where the pH after carbohydrate consumption and the flow of saliva are higher.11 The presence of a fixed retainer in the area does not increase enamel demineralization; rather, it enhances mineralization and the development of a dental calculus.12
The use of fluoride-releasing orthodontic cements could help circumvent this problem, since fluoride ions might deposit directly around bands and brackets, where caries are more likely to develop. However, it should also be noted that the activity of fluoride decreases as pH decreases, and bacterial species such as S. mutans and L. acidophilus decrease the pH in the dental plaque to a value lower than 4.5. The liquid phase of dental plaque is then dehydrated and the loss of hydroxyapatite and fluorapatite crystals occurs, which may never be recovered.10 Therefore, demineralization might occur even with additional fluoride supply.
With this in mind, we investigated the prophylactic potential of selected orthodontic adhesives by determining the concentration of fluoride incorporated into the enamel of rat incisors.
Wistar Wag rats were used as the experimental animal model. The study was approved by the II Local Ethical Committee to Matters of Experiments on Animals at the Medical University of Warsaw, and all procedures complied with its methods of conduct and the international law of protection of animal welfare.
Thirty-six Wistar Wag rats (average weight, 270 g) were randomly divided into 4 groups consisting of 9 rats each. One of the groups received no dental treatment and was used as a control. All other rats were anaesthetized with Nembutal (0.1 mL per 100 g of body weight; Oak Pharmaceuticals Inc., Lake Forest, IL, USA). Bands were cemented to the lower incisors using selected orthodontic adhesives: the resin-modified glass ionomer cement Fuji Ortho SC (GC, Tokyo, Japan), the Illuminate composite (Ortho Organizers Inc., Carlsbad, CA, USA), or the Resilience composite (Ortho Technology Inc., Tampa, FL, USA). All rats were fed with fluoride-free Labofeed H blend; the concentration of fluoride in drinking water was 0.14 mg/L.
Impressions of the lower incisors were taken with individual trays using Phase (Zhermack, Badia Pole sine, Italy) impression material. Metal bands with a height of 5 mm were fitted around the teeth of the obtained plaster models and soldered on. Then, cements were applied according to the manufacturers' recommendations (Figure 1). Before cementation of a band with Fuji Ortho SC, the surface of the teeth was brushed with water and dried, after which the conditioner (10% polyacrylic acid) was put on the enamel surface for 20 seconds. After rinsing with water, the components of the glass ionomer were mixed in proportions indicated by the manufacturer. Before the cementation of a band with Illuminate, the surface of the teeth was treated with 36% phosphate acid, then rinsed with water, dried, and covered with bonding agent. The inner side of each band was coated with the paste before cementation. Illuminate was polymerized for 30 seconds with a halogen lamp. The same procedure was performed for Resilience.
Three rats in each group had their incisors extracted after 6 weeks, and the remaining 6 had their incisors extracted after 12 weeks. Enamel samples were obtained from the areas of interest and fixed to a scanning electron microscopy (SEM) table using graphite glue. Micro analysis of enamel from the buccal surface was performed at the Faculty of Materials Science and Engineering of the Warsaw University of Technology by using a Hitachi S3500N SEM (Hitachi, Tokyo, Japan) equipped with energy dispersive spectrometry (EDS; Thermo Fisher Scientific Inc., Waltham, MA, USA). The analyses were performed in low-vacuum mode to avoid the formation of a conductive coating on the surface of non-conductive samples. Images were taken with a backscattered electrons detector. Both the tooth external surface and specially prepared cross sections were analyzed. For qualitative analysis, an accelerating voltage of 15 kV was applied. The EDS technique is based on the measurement of photon energy emitted by an element after bombarding it with an electron beam. The electrons of a beam penetrated through the sample (at the depth of around 1 µm), and suitable detectors for imaging and analysis of the chemical composition registered the signals. Both qualitative and quantitative analyses were performed. The standard PROZA method was used for quantification of fluoride concentrations in the dental enamel to the depth of 30 µm.13 Representative samples and measurement areas are presented in Figures 2 and 3. Statistical analysis was performed using the STATISTICA (StatSoft, Tulsa, OK, USA) and IDAMS (UNESCO, Paris, France) software.
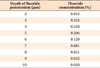
No fluoride ions were found in the enamel of control rats. Six weeks after band cementation, no fluoride penetration to the enamel of the lower incisors was observed in any of the 3 experimental groups. Twelve weeks after band cementation, the enamel of teeth coated with the Resilience composite was still free of fluoride. However, PROZA allowed us to detect fluoride penetration in teeth coated with the Illuminate composite to a depth of 2 µm, which we still consider as being the surface layer of the enamel. In the case of the Fuji Ortho SC cement, PROZA method showed deeper penetration, and further statistical analysis was performed using STATISTICA and IDAMS software. The regression equation y = a + exp × (b + cx) was applied, where exp denotes the exponential function, where the independent variable x was the depth of penetration in µm and the dependent variable y was the content of fluoride ions in %. Parameters of the regression function were estimated with the Levenberg-Marquardt method. The following estimates of the parameter values were obtained: a = -0.018478, b = 0.725329, and c = -0.444921. The statistical significance of the parameters was calculated: for the a parameter, p = 0.8428; for the b parameter, p = 0.0011; and for the c parameter, p = 0.0009. From these results, it was concluded that the a parameter could be eliminated from the model, because its value was not significantly different than 0. A simplified regression equation was thus formulated: y = exp × (0.746117 - 0.465103x). On the basis of this equation, the p-values were p = 0.0002 and p < 0.0001, respectively. The total variance explained by the model amounted to 0.82325. Another issue taken into consideration was the measurement uncertainty analysis. The arithmetic mean of the errors was 0.184 (95% confidence interval, 0.148 - 0.220). The fluoride level and the measurement error were not significantly correlated. The correlation coefficient equaled r = 0.276 with the significance level at p = 0.1545. According to this analysis, the estimated maximal depth of penetration was in the range of 4.8 - 5.7 µm, with the concentration decreasing as the depth increased. The fluoride concentrations at various depths in the enamel of Fuji Ortho S-cemented teeth are shown in Table 1.
In this study, we showed that fluoride released from the resin-modified glass-ionomer cement Fuji Ortho SC penetrated the dental enamel of the rat lower incisors at 12 weeks after orthodontic treatment. These findings support the use of fluoride-releasing orthodontic cements as a prophylactic treatment for caries.
The other 2 orthodontic adhesives tested failed to induce significant fluoride penetration: in the case of Illuminate, fluoride ions reached a depth of 2 µm, thus mainly remaining at the surface of the dental enamel; in the case of Resilience, no fluoride was detected in the enamel.
The penetration of fluoride from resin-modified glass-ionomer Fuji Ortho SC ranged between 4.8 - 5.7 µm, with the concentration decreasing proportionally to the distance from the tooth surface. These values are smaller than those reported in other studies, where the penetration of fluoride from glass-ionomers used as dental fillings reached 20 µm.14 These differences might result from the fact that we applied a very thin layer of adhesive (25 - 35 µm) on the inner side of the bands. However, according to the manufacturer's recommendations, a thin layer of adhesive should be applied to bands and brackets for proper retention. Bonding bands and brackets with excess cement material would fail to function.
In an experimental study conducted by Chin et al.,15 bonding of orthodontic brackets with Fuji Ortho SC resulted in less peribracket enamel demineralization with and without daily fluoride rinsing. The authors concluded that Fuji Ortho SC had a better fluoride-release profile than other adhesives such as Ketac Cem (3M ESPE, Seefeld, Germany), Light-Bond (Reliance Orthodontic Products, Itasca, IL, USA), and Transbond XT (3M Unitek, Monrovia, CA, USA). However, several other factors may affect fluoride absorption. Fluoride is incorporated into apatite crystals during their formation, and fluoride absorption from the environment occurs lifelong. Enamel of recently erupted teeth absorbs more fluoride than matured teeth (so-called posteruptive maturing),16 leaving the former more susceptible to adaptive changes. Another factor is pH: when the pH decreases, the release of fluoride from glass ionomers increases due to chemical erosion and solubility of cement in an acid environment.17 Spherical deposits of CaF2 covered with pellicles of phosphates and proteins break and release fluoride.18 Those ions penetrate into enamel, protecting it and enhancing remineralization.19
If sugars are consumed rarely and oral hygiene is perfect, tooth decay might be slowed down. Demineralization of the enamel during orthodontic treatment can be decelerated when the secretion of saliva is high, minerals are included in the diet, and low amounts of fluoride are provided. It is obvious that introduction of fluoride decreases the frequency of caries and retards its dynamics.20 Research shows that fluoride has a greater effect in reducing the percentage surface coverage (PSC) by S. sanguis biofilms than chlorhexidine.21 Rinsing with 0.05% NaF prior to placement of orthodontic appliances is effective in reducing early biofilm formation.21
However, the use of fixed appliances is usually associated with bad oral hygiene. Extensive demineralization is localized usually gingivally and accompanied by gingivitis. On the surface of enamel, white spots and/or lesions might be observed. The introduction of prophylactic programs and hygiene principles is not always effective because it requires the patient's adherence and willingness to cooperate. However, the average orthodontic treatment time is 2 years. Fluoride-releasing orthodontic adhesives cannot be the only prophylactic agent proposed to patients to prevent caries. Visible erosion around brackets and bands proves that the environment is very cariogenic, and the local supply of fluoride may not be sufficient. Nonetheless, we hypothesize that fluoride penetration from adhesives such as Illuminate and Fuji Ortho SC could increase with time, because the lingual side of teeth does not wear away rapidly and the remodeling of this surface is far from being highly dynamic.
Combining cetylpyridinium chloride, benzalkonium chloride, or chlorhexidine with orthodontic adhesives may enhance defense mechanisms against microbial growth. Indeed, in vitro studies have shown that adding antimicrobial substances decreases accumulation of dental plaque around bands and brackets, reduces the number of bacterial cariogenic colonies, and does not affect bond strengths of orthodontic adhesives.22-24
An alternative to the supply of fluoride to the oral cavity is the introduction of membrane containers with a constant rate of fluoride release. These containers should be bonded to the surface of teeth or appliances. However, if a membrane is broken, an excess dose of fluoride may have toxic effects. Microcapsules with crystalline NaF retained in gingival pockets are also a method for constant fluoride release.25
Figures and Tables
Figure 2
Scanning electron microscope (SEM) imaging of a sample of enamel. SEM allowed quantification of fluoride released from Illuminate at 12 weeks after orthodontic treatment. The rectangle depicts granules of CaF2. Magnification factor, ×350.

References
1. Cueto HI. A little bit of history: the first direct bonding in orthodontia. Am J Orthod Dentofacial Orthop. 1990. 98:276–277.

2. Mitchell DL. The first direct bonding in orthodontia, revisited. Am J Orthod Dentofacial Orthop. 1992. 101:187–189.

3. Newman GV. First direct bonding in orthodontia. Am J Orthod Dentofacial Orthop. 1992. 101:190–191.

4. McComb D. Adhesive luting cements-classes, criteria, and usage. Compend Contin Educ Dent. 1996. 17:759–762.
5. Ogaard B, Rølla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988. 94:68–73.
7. Arneberg P, Ogaard B, Scheie AA, Rölla G. Selection of Streptococcus mutans and lactobacilli in an intra-oral human caries model. J Dent Res. 1984. 63:1197–1200.

8. Rosenbloom RG, Tinanoff N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am J Orthod Dentofacial Orthop. 1991. 100:35–37.

9. Forsberg CM, Oliveby A, Lagerlöf F. Salivary clearance of sugar before and after insertion of fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 1992. 102:527–530.

10. Arneberg P, Giertsen E, Emberland H, Ogaard B. Intra-oral variations in total plaque fluoride related to plaque pH. A study in orthodontic patients. Caries Res. 1997. 31:451–456.

11. Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982. 81:93–98.

12. Mitchell L. Decalcification during orthodontic treatment with fixed appliances--an overview. Br J Orthod. 1992. 19:199–205.

13. Bastin GF, Heijligers HJM. Quantitative electron probe microanalysis of nitrogen. Scanning. 1991. 13:325–342.

14. Wagner L. Fluoride releasing dental filling materials in treatment of caries of deciduous teeth. 2001. Warsaw: Med Tour Press Int..
15. Chin MY, Sandham A, Rumachik EN, Ruben JL, Huysmans MC. Fluoride release and cariostatic potential of orthodontic adhesives with and without daily fluoride rinsing. Am J Orthod Dentofacial Orthop. 2009. 136:547–553.

16. Fejerskov O, Ekstrand J, Burt BA. Fluoride in dentistry. 1996. 2nd ed. Copenhagen: Munksgaard.
17. Rezk-Lega F, Ogaard B, Arends J. An in vivo study on the merits of two glass ionomers for the cementation of orthodontic bands. Am J Orthod Dentofacial Orthop. 1991. 99:162–167.

18. ten Cate JM. Review on fluoride, with special emphasis on calcium fluoride mechanisms in caries prevention. Eur J Oral Sci. 1997. 105:461–465.

19. Ogaard B. CaF(2) formation: cariostatic properties and factors of enhancing the effect. Caries Res. 2001. 35:Suppl 1. 40–44.

20. Morganstein SI. Clinical results: implications for prevention and treatment in general dental practice. Int Dent J. 1994. 44:3 Suppl 1. 297–299.
21. Chin MY, Busscher HJ, Evans R, Noar J, Pratten J. Early biofilm formation and the effects of antimicrobial agents on orthodontic bonding materials in a parallel plate flow chamber. Eur J Orthod. 2006. 28:1–7.

22. Al-Musallam TA, Evans CA, Drummond JL, Matasa C, Wu CD. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am J Orthod Dentofacial Orthop. 2006. 129:245–251.

23. Othman HF, Wu CD, Evans CA, Drummond JL, Matasa CG. Evaluation of antimicrobial properties of orthodontic composite resins combined with benzalkonium chloride. Am J Orthod Dentofacial Orthop. 2002. 122:288–294.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download