Abstract
Objective
The purposes of this study were to measure the palatal soft tissue thickness at popular placement sites of temporary anchorage devices (TADs) by cone-beam computed tomography (CBCT) and evaluate the age, gender, and positional differences in this parameter.
Methods
The study sample consisted of 23 children (10 boys and 13 girls; mean age, 10.87 ± 1.24 years; range, 6.7 to 12.6 years) and 27 adults (14 men and 13 women; mean age, 21.35 ± 1.14 years; range, 20.0 to 23.8 years). Nine mediolateral and nine anteroposterior intersecting reference lines were drawn on CBCT scans of the 50 subjects, and the resultant measurement areas were designated according to their mediolateral (i.e., lateral, medial, and sutural) and anteroposterior (i.e., anterior, middle, and posterior) positions. Repeated-measures analysis of variance was performed to analyze intragroup and intergroup differences.
In the last decade, temporary anchorage devices (TADs) have been merged into the mainstream of clinical orthodontic practice because of several advantages over the conventional anchorage approaches, including simple design of force delivery systems, reduced dependence on patient compliance, and expansion of the existing boundaries of tooth movements.1,2 Their success is affected by various patient factors such as age and the general healing capacity, inflammation status of the periimplant sites, quantity and quality of the cortical bone, and thickness and mobility of the soft tissue in the target areas.3-5
In the oral cavity, the palate is a popular sitefor TAD placement because of the thick keratinized masticatory mucosa, high accessibility, and reduced risk of root damage.6 Recently, several investigators have examined the adjacent areas of the midpalatal suture for use in TAD-assisted distalization mechanics in adolescents and adults.7,8
At present, several methods are available for evaluating the soft tissue thickness of the palate. The use of needles and periodontal probes has been documented since the 1970s.9,10 To avoid the need for local anesthesia, noninvasive techniques involving ultrasonic devices and computed tomography (CT) have also been studied.11-14 However, these approaches have been criticized for either questionable reliability or additional radiation risk.
On the other hand, the accuracy of cone-beam computed tomography (CBCT) for both soft tissue and bone thickness measurements in the maxillary anterior region has recently been confirmed, and a simple technique is now available for assessing the thickness of the palatal masticatory mucosa by CBCT.15-17 However, previous investigations with CBCT were limited to the buccal gingiva or palatal slope area, excluding the paramedian regions of the palate.16-18 Further, the soft tissue thickness at TAD placement sites in the palate at different ages has not been well documented in the literature.
The purposes of this investigation were to measure the palatal soft tissue thickness at popular placement sites of TADs by CBCT and evaluate the age, gender, and positional differences in this parameter.
CBCT scans (i-CAT, Imaging Sciences International, LLC, Hatfield, PA, USA) of randomly selected children (n = 23; 10 boys and 13 girls; mean age, 10.87 ± 1.24 years; range, 6.7 to 12.6 years) and adults (n = 27; 14 men and 13 women; mean age, 21.35 ± 1.14 years; range, 20.0 to 23.8 years) who had visited the Dental Department of Seoul St. Mary's Hospital, The Catholic University of Korea, were collected. The exclusion criteria were the presence of ectopically positioned teeth or pathologic lesions in the palate, previous use of any medication that could affect the oral gingival status such as a calcium channel blocker, and CBCT images with the patient's tongue contacting the palatal soft tissue. The Institutional Review Board of The Catholic University of Korea reviewed and approved the study protocol.
In Vivo Dental (Anatomage Inc., San Jose, CA, USA), a volumetric imaging software, was used for evaluating the palatal soft tissue thickness. Nine anteroposterior (AP) and nine mediolateral (ML) intersecting reference lines forming 81 intersection points were drawn on the CBCT scans. The ML reference lines were drawn at 0, 2, 4, 6, and 8 mm from the midpalatal suture along the coronal plane, and the AP reference lines were drawn at 3-mm intervals from the distal margin of the incisive foramen to 24 mm posteriorly along the sagittal plane (Figure 1).19 The palatal soft tissue thickness was then measured in the sutural (0 mm), medial (2 and 4 mm), and lateral (6 and 8 mm) areas and the anterior (0 - 6 mm), middle (9 - 15 mm), and posterior (18 - 24 mm) areas.
All measurements were performed by one investigator (TV). To test the intra-examiner reliability, 10 randomly selected scans were remeasured 2 weeks later by the same investigator.
SPSS ver. 16.0.2.1 (SPSS Inc., Chicago, IL, USA) was used to analyze the data. In addition to the intraclass correlation (ICC) test, the Bland-Altman method was applied to evaluate the reliability of the assessments.
Because the paired t-test did not show a significant bilateral difference in the soft tissue thickness, only the right side was used for further analysis of the measurement areas. Repeated-measures analysis of variance (ANOVA) was applied to test the intergroup (children vs. adults and male vs. female subjects) and intragroup (ML and AP measurement areas) differences. Statistical significance was determined at p < 0.05.
The ICC coefficient of 0.976 and Bland-Altman plot revealed high intraexaminer assessment reliability (Figure 2).
The gender-based comparison revealed no significant difference (p = 0.124), although the female subjects tended to have thicker palatal soft tissue. Similarly, no significant difference was noted in the age-based comparison (p = 0.309). Interestingly, a significant qualitative interaction between age and the ML position (p = 0.006) was observed, indicating that the children had thicker palatal soft tissue laterally but thinner tissue medially than the adults (Table 1, Figure 3).
The analysis based on the measurement areas showed a significant effect of the ML position (p < 0.001) (Table 2, Figure 4): the lateral and sutural areas had the thickest and thinnest soft tissue, respectively. In contrast, no significant effect of the AP position was noted (p = 0.350) (Table 2). In addition, a significant interaction was observed between the ML and the AP positions (p = 0.011), as shown in Figure 4.
Various techniques are currently available to evaluate the palatal soft tissue thickness.10-17 For instance, Ueno et al.14 demonstrated a high correlation between spiral CT and physical measurements of the maxillary oral mucosa; however, they also recommended that spiral CT should not be used to measure the mucosal thickness alone because of higher radiation exposure. On the other hand, CBCT, which is widely used in contemporary orthodontic practice, ensures a significantly reduced radiation dose due to its lower output and shorter exposure time. Its potential drawbacks include higher image noise and lower contrast resolution from scattered radiation.20
CBCT has recently been applied for reliable measurement of the dentogingival soft tissue.15-17 Januário et al.15 and Barriviera et al.16 used plastic lip retractors and wooden spatulas to retract the soft tissue from the mucosal surface and obtain clear CBCT images. We took similar steps by excluding any scans in which the tongue contacted the roof of the mouth, so that no pressure was applied on the palatal surface and unambiguous measurements could be obtained.
According to our study, the soft tissue over the midpalatal suture was the thinnest, ranging from 1.22 to 1.35 mm, whereas that in the lateral area was the thickest, ranging from 2.51 to 3.00 mm (p < 0.001) (Table 2). Direct comparison with other investigations is somewhat difficult because of the difference in the reference structures. For example, Barriviera et al.16 reported that the mucosal thickness of the higher region of the palate, which may be physically related to the lateral area in our study, ranges from 3.13 to 4.51 mm. Although their slightly elevated data are still within the standard deviations determined in our study, differences in the measurement areas and ethnicity of the patients likely contribute to the discrepancy between the studies.
On the basis of CT images from 100 adult patients, Song et al.13 concluded that the soft tissue of the area over the palatal slope thickens posteriorly, and reported a range of 3.13 to 3.81 mm. However, we could not confirm this AP trend. The difference between their and our data may be explained by the fact that their measurement sites were located further away from the midpalatal suture, where even thicker tissue exists along the palatal slope, because their measurements were performed closer to their own reference structures such as the palatal gingival margins.
Kim et al.18 performed direct measurements of the soft tissue thickness in 23 adult cadavers and found that the thickness along the midpalatal suture stays relatively constant from 1.01 to 0.90 mm beginning 8 mm posterior to the incisive papilla. Consistent with their results, the soft tissue thickness over the suture was stable in our study, ranging from 1.22 to 1.35 mm (Table 2).
Considering age, Wara-aswapati et al.10 used a bone sounding method and discovered that older (mean, 38.7 ± 6.8 years) patients had thicker palatal masticatory mucosa than younger (mean, 16.8 ± 2.0 years) ones (p < 0.01). This difference was not reciprocated in our investigation (p = 0.967). This inconsistency, however, is in agreement with the findings of Eger et al.,11 who also reported no difference among age groups. Possible explanations for these conflicting reports are the difference in the measurement areas and the increased palatal soft tissue thickness in the areas of interest with age. In fact, Song et al.13 reported that the palatal masticatory mucosa is thicker in the age group of 41 to 60 years than in younger and older age groups, clarifying why our investigation did not reveal any significant difference between the children (mean age, 10.87 ± 1.24 years) and the adults (mean age, 21.35 ± 1.14 years).
In terms of gender, we found a general tendency for female subjects to have thicker palatal soft tissue. However, the absence of a significant gender difference (p = 0.491) is inconsistent with the findings of Song et al.13 and Müller et al.,12 who found thinner mucosal tissue in female subjects, but is consistent with those of Cha et al.21 and Wara-aswapati et al.,10 who reported no significant gender difference in the thickness. Many factors may have contributed to these conflicting results, such as differences in the ethnicity of the subjects, reference structures, and measurement techniques, all indicating the need of future investigations with larger sample sizes.
The stability of TADs in the palate depends on both the quantity and the quality of the soft and hard tissues.3,5 Therefore, for maximum retention and minimum inflammation, the desirable profile of a successful TAD placement site is thicker cortical bone underlying thinner attached gingival tissue. The most important finding of this investigation may be that the midpalatal suture and its adjacent areas have thinner soft tissue coverage regardless of gender, age, and the AP position. These results may help clinicians to select the appropriate TAD design, especially for the tissue collar or neck part, for better soft tissue adaptation, reduced inflammation, and greater success. However, they should be interpreted cautiously because of the substantial individual variations in the palatal soft tissue thickness.
CBCT may be a viable tool for assessing the soft tissue thickness in the anterior palatal region. Regardless of age and gender, the soft tissue thins toward the midpalatal suture. Therefore, clinical selection of TAD placement sites should be guided by knowledge of the positional variations in the palatal soft tissue thickness in addition to other contributing factors of TAD stability.
Figures and Tables
Figure 1
Anteroposterior (AP) and mediolateral (ML) reference lines forming 81 intersection points for measuring the palatal soft tissue thickness.
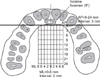
Table 1
Palatal soft tissue thickness (mm) at different measurement areas in adults and children of both genders

References
1. Kinzinger GS, Eren M, Diedrich PR. Treatment effects of intraoral appliances with conventional anchorage designs for non-compliance maxillary molar distalization: a literature review. Eur J Orthod. 2008. 30:558–571.

2. Papadopoulos MA, Tarawneh F. The use of miniscrew implants for temporary skeletal anchorage in orthodontics: a comprehensive review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007. 103:e6–e15.

3. Hoste S, Vercruyssen M, Quirynen M, Willems G. Risk factors and indications of orthodontic temporary anchorage devices: a literature review. Aust Orthod J. 2008. 24:140–148.
4. Reynders R, Ronchi L, Bipat S. Mini-implants in orthodontics: a systematic review of the literature. Am J Orthod Dentofacial Orthop. 2009. 135:564.e1–564.e19.

5. Chen YJ, Chang HH, Huang CY, Hung HC, Lai EH, Yao CC. A retrospective analysis of the failure rate of three different orthodontic skeletal anchorage systems. Clin Oral Implants Res. 2007. 18:768–775.

6. Kyung SH, Lee JY, Shin JW, Hong C, Dietz V, Gianelly AA. Distalization of the entire maxillary arch in an adult. Am J Orthod Dentofacial Orthop. 2009. 135:4 Suppl. S123–S132.

7. Kook YA, Kim SH, Chung KR. A modified palatal anchorage plate for simple and efficient distalization. J Clin Orthod. 2010. 44:719–730.
8. Sandler J, Benson PE, Doyle P, Majumder A, O'Dwyer J, Speight P, et al. Palatal implants are a good alternative to headgear: a randomized trial. Am J Orthod Dentofacial Orthop. 2008. 133:51–57.

9. Greenberg J, Laster L, Listgarten MA. Transgingival probing as a potential estimator of alveolar bone level. J Periodontol. 1976. 47:514–517.

10. Wara-aswapati N, Pitiphat W, Chandrapho N, Rattanayatikul C, Karimbux N. Thickness of palatal masticatory mucosa associated with age. J Periodontol. 2001. 72:1407–1412.

11. Eger T, Müller HP, Heinecke A. Ultrasonic determination of gingival thickness: subject variation and influence of tooth type and clinical features. J Clin Periodontol. 1996. 23:839–845.

12. Müller HP, Schaller N, Eger T, Heinecke A. Thickness of masticatory mucosa. J Clin Periodontol. 2000. 27:431–436.

13. Song JE, Um YJ, Kim CS, Choi SH, Cho KS, Kim CK, et al. Thickness of posterior palatal masticatory mucosa: the use of computerized tomography. J Periodontol. 2008. 79:406–412.

14. Ueno D, Sato J, Igarashi C, Ikeda S, Morita M, Shimoda S, et al. Accuracy of oral mucosal thickness measurements using spiral computed tomography. J Periodontol. 2011. 82:829–836.

15. Januário AL, Barriviera M, Duarte WR. Soft tissue cone-beam computed tomography: a novel method for the measurement of gingival tissue and the dimensions of the dentogingival unit. J Esthet Restor Dent. 2008. 20:366–373.

16. Barriviera M, Duarte WR, Januário AL, Faber J, Bezerra AC. A new method to assess and measure palatal masticatory mucosa by cone-beam computerized tomography. J Clin Periodontol. 2009. 36:564–568.

17. Fu JH, Yeh CY, Chan HL, Tatarakis N, Leong DJ, Wang HL. Tissue biotype and its relation to the underlying bone morphology. J Periodontol. 2010. 81:569–574.

18. Kim HJ, Yun HS, Park HD, Kim DH, Park YC. Soft-tissue and cortical-bone thickness at orthodontic implant sites. Am J Orthod Dentofacial Orthop. 2006. 130:177–182.

19. Moon SH, Park SH, Lim WH, Chun YS. Palatal bone density in adult subjects: implications for mini-implant placement. Angle Orthod. 2010. 80:137–144.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download