Abstract
Objective
To evaluate the short-term effect of fangchinoline, an antiinflammatory drug widely used in Asia, on root resorption that is associated with orthodontic tooth movement.
Methods
Twenty-four Wistar rats were randomly divided into 6 groups. Mesial forces of 0, 50, or 100 g were applied to the maxillary first molar of the rats in each group for 14 days by activating nickel-titanium closed-coil springs. One-half of the rats receiving each of these treatments also received injections of 200 µL fangchinoline every 2 days. Finally, movement of the maxillary first molars was measured using digitized radiographs. The molars were extracted and the surfaces of the root resorption craters were recorded using a scanning electron microscope. The distance the molars moved and resorptionarea ratio was measured, and results were analyzed using 2-way ANOVA tests.
Results
There were no statistical differences in the distances the first molars moved under 50 or 100 g force, regardless of treatment with fangchinoline. However, the resorption area ratios were significantly smaller in those rats that were treated with both tension and fangchinoline than in those rats treated by tension alone.
Orthodontically induced root resorption (OIRR) is a sequela of orthodontic treatment that affects nearly 100% of treated patients.1 OIRR can induce cementum and dentin damage, depending on its severity. Although the normal root structure might later be partially repaired by cementum, severe resorption can destroy it, leading to the failure of orthodontic treatment. Because of its harm to dental health, great efforts have been made to identify mechanotherapies that might reduce or eliminate root resorption.
OIRR is related to total apical displacement, treatment duration, age, gender, malocclusion type, root shape, type of appliance, treatment modality, and force regimen.2 However, a hypofunctional periodontium may also contribute to OIRR.3 The primary cells responsible for this process are the odontoclasts.4 A group of proinflammatory factors and enzymes are involved in the differentiation, maturation, and activation of these cells.5 Increased knowledge of this process has caused investigators to focus on certain inhibitors as potential treatments. Fangchinoline (C37H40N2O6) is an alkaloid that is extracted from bisbenzylisoquinoline and is widely used in Asian countries as an anti-inflammatory drug. Fangchinoline acts through more mechanisms, and is safer, than other anti-inflammatory agents.
Fangchinoline inhibits 90% of interleukin (IL)-1 and tumor necrosis factor-alpha (TNF-α) production at a dose of 10 µg/mL.6 Fangchinoline also partially inhibits cyclooxygenase and human IL-6,7 is a nonspecific calcium channel inhibitor,8,9 and has an antithrombosis function.10 At the same time, fangchinoline has effective antioxidant and radical-scavenging activity.11 Our laboratory recently found fangchinoline to be a potential cathepsin K (CK) inhibitor. Fangchinoline is an excellent candidate for inhibiting root resorption because it is safe and has multiple anti-inflammatory effects.
The aim of this study was to examine the effects of fangchinoline on root resorption during tooth movement. We hypothesized that fangchinoline would limit root resorption during orthodontic tooth movement.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the local district government and the Animal Care Commissioner of Jilin University.
We used an online sample-size calculator for clinical trials and scientific experiments (hedwig.mgh.harvard. edu) to determine the appropriate sample size for a study with a statistical power of 0.8. Twenty-four 8 week old male Wistar rats, with an average weight of 200 g, were purchased from the Animal Center of Jilin University. Rats were randomly allocated to 1 of 6 control or treatment groups. There were 4 rats in each group. Rats in the treatment groups had either 50 g or 100 g of orthodontic force applied to their maxillary right first molars, while control rats were not subjected to orthodontic force (0 g). Treatment and control groups were further divided into 2 subgroups, based on whether they received fangchinoline injections. Rats in the 0 g(+), 50 g(+), and 100 g(+) groups received 40 µL of 5 µg/µL fangchinoline every 2 days. Rats in the 0 g(-), 50 g(-), and 100 g(-) groups received no fangchinoline.
We based the dose of fangchinoline on a study by Onai et al.6 and the unpublished results of our earlier study. All of the rats were observed for 7 days prior to beginning the experiment, to ensure that they were healthy. Rats were fed a soft diet during the 14 days experiment.
Orthodontic appliances were installed on the rats while under anesthesia with SuMianXin (Institute of Military Medicine Science, Changchun, China) (0.2 mL/kg) IM. The appliances consisted of a nickel-titanium coil spring (ShengMaTe Co., Shanghai, China) tied between the maxillary incisors and right first molar (Figure 1). The force of the springs was measured using an ergometer before installing them. We covered the end of the ligature wire with a piece of resin in order to prevent appliance loosening.
In our experiment, 200 µg fangchinoline was given every 2 days. Twenty milligrams fangchinoline (YuanYe Co., Shanghai, China) was added to 4 mL distilled water, forming an injectable suspension with a concentration of 5 µg/µL. We injected 40 µL fangchinoline every 2 days into the subperiosteum adjacent to the maxillary first molar of the rats in the 0 g(+), 50 g(+), and 100 g(+) groups. The suspension was stored at 4℃ and stirred before each injection.
All rats were killed by decapitation at the end of the experiment. After 14 days of force application, the amount of tooth movement was measured on cephalometric radiographs, as previously reported.12 The maxillary right first molar and the adjacent alveolar bone was extracted in toto for further examination. All tissues were soaked in 5% sodium hypochlorite solution for 12 hours, at which time the alveolar bone was delicately removed, exposing the 5 roots of the first molar. The periodontal ligament residue of the distobuccal and distopalatal roots were then removed and carefully cleaned to reveal clear root surfaces. All teeth were dried and observed under an SEM (JSM-6390A; JEOL Ltd., Tokyo, Japan) at the same distance and orientation. Images of the mesial surfaces of both distal roots were saved as digital photographs.
The distance between the contact points of the first and second molar in each rat was measured using ImageJ software (version 1.44; National Institutes of Health, USA). The resorption area and area of the entire mesial surface of the 2 distal roots were measured separately using the same software. We obtained the resorption-area ratio by dividing the crater area by the total surface area (Figure 2). Each measurement was made 3 times by the same examiner, and the mean of these values was used in further analyses. We performed a 2-way ANOVA to compare the paired treatment groups using SPSS version 17 (SPSS Inc., Chicago, IL, USA).
None of the rats had any distance between the first and second molar crown prior to the experiment. Tooth movement occurred in all of the rats in the treatment groups by the end of the experiment (Table 1). No force was loaded on the rats in either control group and no movement had occurred. Statistical analysis indicated that the distance of tooth movement in the 50 g and 100 g groups was significantly greater than that in the 0 g group (p < 0.05). However, there was no significant difference in the distance of tooth movement between the 50 g and 100 g groups, irrespective of fangchinoline injection (p > 0.05).
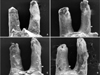
Root resorption was not detected in the control group, and smooth cementum could be seen on the mesial surface of the distobuccal and distopalatal roots. However, root resorption was observed among the treatment group rats, mainly in the cervical and middle one-third of the root (Figure 3). There was no significant difference between the 50 g(-) group and the 100 g(-) group in resorption area ratio. However, the resorption area ratio was less severe in the fangchinoline treatment groups than in the non-fangchinoline treatment groups, irrespective of force magnitude (p < 0.05) (Table 2).
In this study, we used SEM and X-ray to quantify root resorption and tooth movement. After 14 days, the maxillary first molar moved no further in the 50 g(+) and 100 g(+) groups than it did in the 50 g(-) and 100 g(-) groups. These results agree with those of Gonzales et al.,12 indicating that there is no significant difference in tooth movement after application of force at these magnitudes. Rat tooth movement is divided into 3 phases: an initial phase lasting 2 days, a lag phase, and a final linear increment of tooth movement.13,14 In our study, tooth movement over the 14 days was relatively slow and was the result of the teeth shifting in the periodontal ligament space. Because odontoclasts and osteoclasts are similar, we expected that when the same amount of force was applied, there would be more tooth movement in the fangchinoline group than in the non-fangchinoline group. However, we found no difference between the tooth movement of groups that received fangchinoline and those that did not. This is may be partially due to a lag in tooth movement caused by hyalinization. Additionally, there is still no consensus regarding the relationship between root resorption and bone resorption; therefore, further research is necessary in this regard.15 Experience with human patients indicates that molars have a tendency to move toward open spaces along the arch. We assumed this would also apply to rats: the second molar would move forward after the movement of the first molar. Because this process takes time to occur, we did not expect any movement of the second molar within the 14 day experimental period. However, this effect should be taken into account in studies with longer treatment periods.
Several researchers have attempted to investigate the precise mechanisms of root resorption. Orthodontic movement is dependent on bone resorption on the pressure side and odontoclasts are now considered to be responsible for root resorption. Odontoclasts are thought to originate from circulating progenitor cells and have characteristics that are similar to osteoclasts, such as expression of CK, cathepsin D, matrix metalloproteinase-9, H+-ATPase, and others. The activation of odontoclasts is regulated by the nuclear factor kappa B/nuclear factor kappa B ligand/osteoprotegerin (RANK/RANKL/OPG) pathways.16,17
In the present study, there was no significant difference in the resorption area ratio of 50 g(-) rats and 100 g(-) rats. This result parallels those of Zhuang et al.,18 indicating that force level is not the sole contributor to root resorption. Resorption areas in the 50 g(+) and 100 g(+) groups were smaller than in the 50 g(-) and 100 g(-) groups, respectively, supporting the hypothesis that fangchinoline inhibits root resorption. CK is a cysteine protease that is responsible for the degradation of collagen and matrix proteins.19 CK inhibitor has been investigated as a potential target for osteoporosis treatment.20 Our past research indicated that fangchinoline is a CK inhibitor; therefore, we assumed that this inhibitory effect would also apply to resorption. Moreover, fangchinoline partly inhibits IL-1 and TNF-α, which may also explain its inhibitory effect on root resorption. A study by Jäger et al.21 indicated that systemic application of soluble receptors to IL-1 and TNF-α reduces the number of odontoclasts, lending further support to our hypothesis that fangchinoline would limit root resorption during orthodontic tooth movement.
Because root resorption craters are usually 3-dimensional, further investigation is needed to determine the effect of fangchinoline on crater depth, as well as its influence on the various phases of tooth movement.
Figures and Tables
Figure 1
Rat tooth movement model. A nickel-titanium coil spring was activated after insertion between the first molar and incisors in order to move the molar mesially.
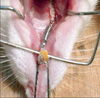
Figure 2
Calculation of the resorption area ratio in the molars of Wistar rats. A, Representative scanning electron microscope micrograph of the mesial surface of the distopalatal root. B, Diagram indicating the area of the crater (black) as compared to the total root surface area (gray). The resorption area ratio was calculated by dividing the area of the crater by the total root surface area (resorption area ratio = black area/gray area).
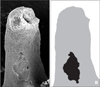
Figure 3
Representative scanning electron microscope micrographs of the distal root surface of molars from Wistar rats. A, 50 g force and no fangchinoline; B, 50 g force with injection of 40 µL of 5 µg/µL fangchinoline every 2 days; C, 100 g force and no fangchinoline; D, 100 g force with injection of 40 µL of 5 µg/µL fangchinoline every 2 days. Different sizes of resorption craters can be observed on the mesial surface of the root image from each group. Craters are found primarily on the cervical and middle one third of each root (white arrow).
Notes
References
1. Brudvik P, Rygh P. Non-clast cells start orthodontic root resorption in the periphery of hyalinized zones. Eur J Orthod. 1993. 15:467–480.

2. Segal GR, Schiffman PH, Tuncay OC. Meta analysis of the treatment-related factors of external apical root resorption. Orthod Craniofac Res. 2004. 7:71–78.

3. Sringkarnboriboon S, Matsumoto Y, Soma K. Root resorption related to hypofunctional periodontium in experimental tooth movement. J Dent Res. 2003. 82:486–490.

4. Harokopakis-Hajishengallis E. Physiologic root resorption in primary teeth: molecular and histological events. J Oral Sci. 2007. 49:1–12.

5. Meikle MC. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur J Orthod. 2006. 28:221–240.

6. Onai N, Tsunokawa Y, Suda M, Watanabe N, Nakamura K, Sugimoto Y, et al. Inhibitory effects of bisbenzylisoquinoline alkaloids on induction of proinflammatory cytokines, interleukin-1 and tumor necrosis factor-alpha. Planta Med. 1995. 61:497–501.

7. Choi HS, Kim HS, Min KR, Kim Y, Lim HK, Chang YK, et al. Anti-inflammatory effects of fangchinoline and tetrandrine. J Ethnopharmacol. 2000. 69:173–179.

8. Kim HS, Zhang YH, Oh KW, Ahn HY. Vasodilating and hypotensive effects of fangchinoline and tetrandrine on the rat aorta and the stroke-prone spontaneously hypertensive rat. J Ethnopharmacol. 1997. 58:117–123.

9. Zhang YH, Fang LH, Ku BS. Fangchinoline inhibits rat aortic vascular smooth muscle cell proliferation and cell cycle progression through inhibition of ERK1/2 activation and c-fos expression. Biochem Pharmacol. 2003. 66:1853–1860.

10. Kim HS, Zhang YH, Yun YP. Effects of tetrandrine and fangchinoline on experimental thrombosis in mice and human platelet aggregation. Planta Med. 1999. 65:135–138.

11. Gülçin I, Elias R, Gepdiremen A, Chea A, Topal F. Antioxidant activity of bisbenzylisoquinoline alkaloids from Stephania rotunda: cepharanthine and fangchinoline. J Enzyme Inhib Med Chem. 2010. 25:44–53.

12. Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 2008. 78:502–509.

13. Reitan K, Kvam E. Comparative behavior of human and animal tissue during experimental tooth movement. Angle Orthod. 1971. 41:1–14.
15. Wang Z, McCauley LK. Osteoclasts and odontoclasts: signaling pathways to development and disease. Oral Dis. 2011. 17:129–142.

16. Oshiro T, Shibasaki Y, Martin TJ, Sasaki T. Immunolocalization of vacuolar-type H+-ATPase, cathepsin K, matrix metalloproteinase-9, and receptor activator of NFkappaB ligand in odontoclasts during physiological root resorption of human deciduous teeth. Anat Rec. 2001. 264:305–311.

17. Tsuji Y, Yamaza T, Kido MA, Goto T, Nakata S, Akamine A, et al. Expression of cathepsin K mRNA and protein in odontoclasts after experimental tooth movement in the mouse maxilla by in situ hybridization and immunoelectron microscopy. Cell Tissue Res. 2001. 303:359–369.

18. Zhuang L, Meng X, Li P, Bai Y. A pilot study on three dimensional morphology of root by Micro-CT during orthodontic root resorption. J Modern Stomatol. 2010. 24:35–38.
19. Inaoka T, Bilbe G, Ishibashi O, Tezuka K, Kumegawa M, Kokubo T. Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun. 1995. 206:89–96.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download