Abstract
Purpose
Rome criteria are considered the gold standard for diagnosing functional constipation. The modified Bristol stool form scale (m-BSFS) was validated to measure stool form in children. However, neither the potential use of the m-BSFS as a tool to facilitate the diagnosis of potential constipation, nor the agreement between m-BSFS and stool consistency by Rome has been studied. Our objective is to determine if m-BSFS is a reliable tool to facilitat detection of constipation; and the agreement between stool form by m-BSFS and hard stool criteria in Rome.
Methods
A survey tool with the Rome III criteria and the m-BSFS was developed. A Likert-scale addressed frequency of each stool form on the m-BSFS. Responses to Rome III and m-BSFS were compared.
Results
The sensitivity and specificity of the m-BSFS was 79.2% and 66.0% respectively; and in children <4 years. improved to 81.2% and 75.0% respectively. There was poor agreement between hard stools by m-BSFS and the painful or hard bowel movement question of Rome Criteria.
Conclusion
The potential utility of m-BSFS as a reasonably good tool to facilitate the diagnosis of potential constipation in children is shown. The poor agreement between painful or hard stool question in Rome III, and ratings for hard stool on the m-BSFS illustrates that one's perception may differ between a question and a picture. A useful pictorial tool to appraise stool form may, thus, be a favorable complement in the process of enquiry about bowel habits in well-child care.
Constipation is a common ailment in childhood, with a prevalence ranging from 5% to 30% [1]. It accounts for 3% of all Pediatric clinic visits and a quarter of visits to Pediatric Gastroenterology clinics [2]. The most common type occurring in children is functional constipation- a state in which there is no apparent underlying disease process [3]. The Rome criteria—consisting of history and physical examination—based criteria are the gold standard for diagnosis. Constipation is under reported, or it may be diagnosed when severe complications arise, such as rectal bleeding, chronic rectal pain, stool incontinence, or recurrent abdominal pain [45]. Children with functional constipation, in both self and parent reports, have consistently reported low health-related quality of life, even in comparison to children suffering from inflammatory bowel disease, cancer, and liver transplant [67].
Chronic constipation also entails sizable healthcare costs [8]. A recent study evaluating the emergency department burden of constipation in the United States between 2006–2011 derived from the Nationwide Emergency Department Sample database identified 1.6 billion dollars spent on all constipation-related emergency department visits in 2011. Infants and children had particularly high rates of constipation-related emergency department visits [9]. Early detection of constipation may prevent chronicity and associated complications [1011].
While constipation may be attributed to different symptoms, including, but not limited to- reduced frequency, hard stools, straining, or a sense of incomplete evacuation, in routine clinical practice, enquiry about “elimination concerns” is mostly limited to stool frequency, and tends to ignore stool consistency [312]. The Rome criteria, which are the gold standard for diagnosing functional constipation, include a criterion regarding stool consistency [13].
The European & North American Societies for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN and NASPGHAN) in their most recent evidence-based recommendations have also stressed the importance of enquiry about stool consistency in the evaluation of constipation in children [14]. Stool form (consistency) correlates well with gut transit time, which is delayed in children with functional constipation [15161718]. The modified Bristol stool form scale (m-BSFS) was developed to assess stool form in children. It has shown a high degree of inter-rater reliability, intra-rater reliability, and agreement among pediatric gastroenterologists [192021].
Therefore, this study was undertaken to explore the value of the m-BSFS as a simple, easy-to-use tool for objective evaluation of bowel habits in healthy children presenting for preventive health visits. To this end, we compared responses to the m-BSFS against responses to a written questionnaire based on the Rome III criteria.
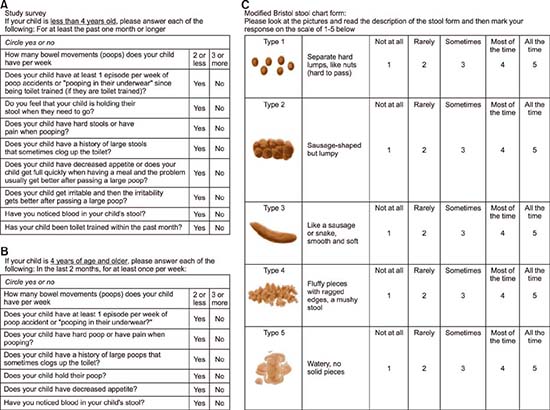
We created and piloted a survey instrument combining an adaptation of the Rome III criteria (for age <4 years. and age >4 years. as described by Rome III), and the m-BSFS linked to a 5 point-Likert scale representing the relative frequency of each stool type, with symptoms present over the past 2 months. See Survey Instrument in Appendix 1. For the Rome III-based questionnaire, a patient was designated as meeting criteria for possible functional constipation if the parent or patient answered, “yes” to two or more of the major criteria, the first 5 questions in that part of the survey. The remaining questions were minor criteria included for completeness. For the m-BSFS, we looked at the stool forms that were reported to occur at the highest frequency on the Likert scale. Patients were designated as having hard stools if the frequency score for either type I or type II stools (the hardest stools) was greater than or equal to the highest frequency score for any of the other three types of stool on the chart. The question in the Rome III-based questionnaire that corresponds with stool consistency was “Does your child have hard poop or pain on pooping?”
Our research assistant (RA) approached families arriving consecutively in the waiting room of a large ambulatory pediatric clinic associated with a county hospital in a Northeastern Ohio city. Inclusion criteria were children presenting for preventive health visits and families proficient in English. Families presenting for urgent care visits, and children with special health care needs like cerebral palsy and neurological diseases were excluded. After obtaining verbal consent, the RA handed the survey to the family. When two parents were present the RA obtained consent from the mother and asked her to complete the forms. Many mothers were observed to solicit input from their children, depending on the child's age. There were a few adolescents that chose to answer the survey independently. When more than one child was present, the RA asked the parent to complete the questionnaires with reference to the child who was there for the clinic visit. The RA offered to read the forms aloud to parents; otherwise, parents were expected to respond to the forms without assistance. The survey responses were anonymous. Data collection proceeded over a period of 4 weeks. The surveys were not shown to the primary care doctors, who carried out their usual visits oblivious of the results of the questionnaires. Indeed, the primary care doctors were largely unaware that the study was taking place in the waiting room. The study protocol was approved by the IRB at Metro-Health Medical Center, Case Western Reserve University.
All of the analysis were performed on a complete-case basis. The m-BSFS was assessed for sensitivity and specificity compared to responses of the Rome-based questionnaire. The 95% confidence intervals (CIs) were calculated using standard practices. A Kappa agreement test was run to assess the agreement between hard stool consistencies on m-BSFS and the Rome III criteria responses for painful or hard stools. The association between the designation of criteria met for functional constipation (potential) based on the Rome criteria and hard stools per the m-BSFS was measured with a Phi score.
In total, 371 patients were approached for participation; of these 59 declined to participate, 69 weren't there for a well-child visit, 30 weren't proficient in English, and 4 were excluded from final analysis due to non-completion of survey, leaving a final sample size of 209. The parent and child together completed 199 of these; adolescent aged subjects completed 10 independently, see Fig. 1. The demographic characteristics and frequency of symptoms in our study population are outlined in Tables 1 and 2, respectively.
In our study, 23.0% (48/209) of all children met criteria for potential functional constipation according to the Rome III based survey. Exactly 15.0% (16/107) of children <4 years, versus 31.4% (32/102) of children >4 years. met criteria for potential functional constipation (p=0.0037). Exactly 44.5% (93/209) of all children had frequent hard stools according to the m-BSFS (see Survey development under MATERIALS AND METHODS). Exactly 33.6% (36/107) of children <4 years, versus 54.9% (56/102) of children >4 years had frequent hard stools according to the m-BSFS (p=0.0012).
We noted an association between the designation of meeting criteria for functional constipation based on the Rome criteria and frequent hard stools per the m-BSFS with a Phi score of 0.3809 (p<0.001).
Using the Rome-based questionnaire as our gold standard for the designation of criteria met for potential functional constipation, the sensitivity and specificity of the m-BSFS 79.2% (95% CI, 65.0–89.5) and 66.0% (95% CI, 58.0–73.1) respectively. The positive predictive value was 40.9% (95% CI, 30.9–51.6) and the negative predictive value was 91.4% (95% CI, 84.3–95.6).
For children <4 years, the sensitivity and specificity further improved to 81.2% (95% CI, 54.3–95.9) and 75.0% (95% CI, 64.5–83.2), respectively. The positive predictive value was 44.6% (95% CI, 31.6–58.4) and the negative predictive value was 84.1% (95% CI: 69.3–92.8), see Table 3.
The most important finding of our study is that the m-BSFS has reasonable value as a simple, quick, and easy-to-use tool to objectively evaluate stool form in children, as compared against a more extensive questionnaire based on the Rome criteria. The performance characteristics of the m-BSFS were particularly strong in younger children. Even though the m-BSFS alone can't be used for the diagnosis of constipation, however, parent/patient responses to frequent hard stools should alert the physician to the presence of potential constipation.
During routine preventive visits for well child care, the Bright Future Guidelines recommend bowel movement enquiry by simply asking ‘Does your child have any elimination concerns?’ [2223]. Simply asking this one question may be insufficient for the enquiry of possible constipation. In our review of randomly selected charts, the Rome criteria weren't being routinely documented in the diagnosis of constipation either. This observation suggests that bowel habits suggestive of possible constipation is under-reported and, hence, possibly underdiagnosed [4524].
While guidelines exist for the diagnosis of functional constipation in children, there are no official recommendations for screening for constipation in children. Wald et al. [24] screened children aged 5–8 years. for constipation using a bowel-habit questionnaire and found a sensitivity of 59.6% and a specificity of 82.6%, as compared to a bowel habit diary; they concluded that the sensitivity needed to be improved before using their tool as a routine screening tool [25]. Our results using the m-BSFS to assess stool form have yielded higher sensitivity and specificity vis-à-vis a questionnaire-based assessment, especially in younger children. This may be because parents of younger children are more likely to be changing diapers, and thus better able to give accurate answers about their child's stool form. Stool form, not stool frequency, has shown good correlation with whole gut transit times both in adults and children- and prolonged gut transit times are implicated in chronic constipation [151626].
However, in our experience, enquiry about “elimination” in well-child clinics is mostly limited to stool frequency, i.e., “Does your child poop regularly?” Over-reliance on stool frequency as a surrogate marker for constipation may thus contribute to under-identification of constipation in primary care. This pitfall is further highlighted by the findings in a population-based study conducted in Sri Lanka, where stool habits of 2,273 children were analyzed. In that study, children who complained of “hard” stool reported higher stool frequency (7.28 per week) than children who reported normal stool consistency (6.89 stools per week) [27]. A child who passes “rabbit turds” daily is not evacuating his bowels effectively and may get an urge to defecate frequently. Simply asking about “hard stool” however may not provide comparable information. We did not find significant agreement between the painful or hard stool question on our Rome-based questionnaire and the responses of hard stool form on m-BSFS. We speculate that the words “hard” and “painful” may be more open to individual interpretation than are the visual images of stools. Moreover, not all hard stools may necessarily be painful. Thus, a reliable pictorial tool to appraise stool form may be a favorable complement in the process of enquiry about bowel habits in children.
We would like to highlight the strengths of our study. Our RA enrolled a consecutive sample of children who presented for preventive health visits. He also offered assistance to families who needed help. We designed our questionnaire based on Rome which is the accepted gold standard for diagnosis of functional constipation. Our study also suggests a novel method for interpreting the m-BSFS. One of the criticisms for the use of the BSFS has been a lack of consensus about how to interpret it for individual patients. For example, a patient may point to more than one stool form during the clinic visit. Russo et al. averaged the BSFS scores to achieve a mean stool form, but it is unclear how well an average of one's stool form reflects the day-to-day variability of stool form [27]. In our study, we used a Likert scale to gauge the frequency of each individual stool form.
We believe that this is a more meaningful way to interpret the responses of the m-BSFS. Using this scoring method, the m-BSFS was significantly associated with the results of our Rome-related questionnaire, supporting the utility of this approach.
We acknowledge some limitations of our study. We used a questionnaire based on Rome III as a benchmark for assessing the usefulness of m-BSFS, rather than in person diagnosis by a doctor; our study design precluded the physician evaluation component. Thus, the extent to which our questionnaire agrees with expert diagnosis is unknown. Hence the true prevalence of constipation is unknown. However, the fact that our observed prevalence of patients who met Rome based survey criteria for possible functional constipation of about 23% falls roughly in the middle of published estimates (5–30%). This suggests that our use of the questionnaire based on Rome criteria was reasonable. Also, the use of the Rome criteria in a survey format for infant and toddler functional gastrointestinal disorders has been validated in other studies [2028].
Lastly, the anonymous nature of this study prevented direct association between the survey results and the clinical diagnosis for an individual patient.
Our study is the first to show the potential value of m-BSFS as a reasonably good tool for objectively evaluating bowel habits of children during preventive health visits. While the m-BSFS can not be used as a sole criterion for the diagnosis of constipation, patient ratings of frequent hard stool forms should alert the physician to the presence of possible constipation. Physicians should then perform a complete diagnostic evaluation for constipation.
The poor kappa agreement between the question for painful or hard stool and the ratings for hard stool on the m-BSFS illustrates that one's perception of hard stool may differ between a questions and when one is shown a picture, or the fact that not all hard stools may necessarily be painful. A reliable pictorial tool to appraise stool form may, thus, be a favorable complement in the process of enquiry about bowel habits in children.
Future research to validate the m-BSFS as a screening tool should be pursued in the form of studies in which findings from the m-BSFS could be compared to an expert clinical assessment for functional constipation, as well as studies documenting long-term benefits of early identification of constipation.
Figures and Tables
Table 1
Comparison of Demographic Features between Children Less Than Four Years Old and Greater Than Four Years Old Groups

ACKNOWLEDGEMENTS
The authors would like to thank MetroHealth Medical Center, in particular the Department of Pediatrics and Gastroenterology for their support.
References
1. van den Berg MM, Benninga MA, Di Lorenzo C. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 2006; 101:2401–2409.

2. Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 2011; 25:3–18.
3. Baker SS, Liptak GS, Colletti RB, Croffie JM, Di Lorenzo C, Ector W, et al. Constipation in infants and children: evaluation and treatment. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 1999; 29:612–626.
4. Uc A, Hyman PE, Walker LS. Functional gastrointestinal disorders in African American children in primary care. J Pediatr Gastroenterol Nutr. 2006; 42:270–274.

5. Burgers R, Bonanno E, Madarena E, Graziano F, Pensabene L, Gardner W, et al. The care of constipated children in primary care in different countries. Acta Paediatr. 2012; 101:677–680.

6. Youssef NN, Langseder AL, Verga BJ, Mones RL, Rosh JR. Chronic childhood constipation is associated with impaired quality of life: a case-controlled study. J Pediatr Gastroenterol Nutr. 2005; 41:56–60.

7. Rappaport L, Landman G, Fenton T, Levine MD. Locus of control as predictor of compliance and outcome in treatment of encopresis. J Pediatr. 1986; 109:1061–1064.

8. Liem O, Harman J, Benninga M, Kelleher K, Mousa H, Di Lorenzo C. Health utilization and cost impact of childhood constipation in the United States. J Pediatr. 2009; 154:258–262.

9. Sommers T, Corban C, Sengupta N, Jones M, Cheng V, Bollom A, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015; 110:572–579.

10. Bongers ME, van Wijk MP, Reitsma JB, Benninga MA. Long-term prognosis for childhood constipation: clinical outcomes in adulthood. Pediatrics. 2010; 126:e156–e162.

11. Loening-Baucke V. Constipation in early childhood: patient characteristics, treatment, and longterm follow up. Gut. 1993; 34:1400–1404.

12. Arbuckle RA, Carson RT, Abetz-Webb L, Hyams J, Di Lorenzo C, Lewis BE, et al. Measuring the symptoms of pediatric constipation and irritable bowel syndrome with constipation: expert commentary and literature review. Patient. 2014; 7:343–364.

13. Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, et al. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014; 58:258–274.

14. Carmo RL, Oliveira RP, Ribeiro AE, Lima MC, Amorim BJ, Ribeiro AF, et al. Colonic transit in children and adolescents with chronic constipation. J Pediatr (Rio J). 2015; 91:386–391.

15. Zaslavsky C, da Silveira TR, Maguilnik I. Total and segmental colonic transit time with radio-opaque markers in adolescents with functional constipation. J Pediatr Gastroenterol Nutr. 1998; 27:138–142.

16. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997; 32:920–924.

18. Chumpitazi BP, Lane MM, Czyzewski DI, Weidler EM, Swank PR, Shulman RJ. Creation and initial evaluation of a Stool Form Scale for children. J Pediatr. 2010; 157:594–597.

19. van Tilburg MA, Rouster A, Silver D, Pellegrini G, Gao J, Hyman PE. Development and validation of a Rome III functional gastrointestinal disorders questionnaire for infants and toddlers. J Pediatr Gastroenterol Nutr. 2016; 62:384–386.

20. Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. Reliability and validity of a modified Bristol Stool Form Scale for children. J Pediatr. 2011; 159:437–441.

21. Loening-Baucke V. Prevalence rates for constipation and faecal and urinary incontinence. Arch Dis Child. 2007; 92:486–489.

22. Geoffrey RS, Cynthia B, Graham AB 3rd, Brown OW, Hardin A, Lessin HR, et al. 2014 recommendations for pediatric preventive health care. Pediatrics. 2014; 133:568–570.

23. Hagan JF, Shaw JS, Duncan PM. Bright futures: Guidelines for health supervision of infants, children, and adolescents. American Academy of Pediatrics;2007.
24. Wald ER, Jagodzinski TD, Moyer SC, Wald A, Eickhoff JC, Edmonson MB. Validation and clinical utility of a bowel habit questionnaire in school-age children. J Pediatr Gastroenterol Nutr. 2011; 53:520–523.

26. Devanarayana NM, Rajindrajith S. Bowel habits and behaviors related to defecation in 10- to 16-year-olds: impact of socioeconomic characteristics and emotional stress. J Pediatr Gastroenterol Nutr. 2011; 52:569–573.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download