Abstract
Purpose
Minimal change esophagitis (MCE) is a reflux disease without mucosal breaks, known to be partially associated with abnormal gastric motor function. Electrogastrography (EGG) is commonly applied to assess gastric motor function in a noninvasive fashion. We aimed to determine the relationship between MCE and gastric myoelectrical activity (GME) recorded on EGG in children.
Methods
We retrospectively assessed the records of 157 children without underlying disease who underwent both EGG and upper gastrointestinal endoscopy at Gachon University Gil Medical Center between January 2010 and June 2015. The children were stratified according to the appearance of the esophagus (normal vs. MCE). Between-group differences in EGG parameters and their correlation with each MCE finding were statistically analyzed.
Results
Only the power ratio, one of the EGG parameters analyzed, differed significantly between the two groups (MCE, 1.68±3.37 vs. normal, 0.76±1.06; p<0.05), whereas the other parameters, such as dominant frequency, dominant power, and the ratio of abnormal rhythm, showed no differences. Among children with MCE, significant correlations were noted between erythema and power ratio (p<0.05), friability and postprandial dominant frequency (p<0.05), and edema and/or accentuation of mucosal folds and pre-prandial frequency (p<0.05). Helicobacter pylori infection correlated with postprandial arrhythmia (MCE, 33.59±15.52 vs. normal, 28.10±17.23; p<0.05). EGG parameters did not differ between children with normal esophagus and those with biopsy-proven chronic esophagitis.
According to the guidelines put forth by the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition, gastroesophageal reflux represents the passage of gastric contents into the esophagus, with or without regurgitation and vomiting [1]. While gastroesophageal reflux is a normal physiological phenomenon in children, its aggravation or progression can lead to gastroesophageal reflux disease (GERD) and its associated symptoms [2]. The prevalence of GERD with heartburn and/or acid regurgitation symptoms lasting for at least one week is 10-20% across all age groups in western countries, whereas lower rates have been reported in Asia [3]. Meanwhile, the prevalence of GERD in children has not been fully estimated and varies according to age and country. In Japan, the prevalence of GERD is 4.4% in individuals younger than 20 years, and 11.6% in those older than 20 years [4]. While there have been no population-based studies in Korea, a previous report indicated that 19.9% of children who visited a tertiary hospital due to upper abdominal pain were diagnosed with GERD [5].
According to the Los Angeles classification, GERD is classified as being of erosive type (ERD) or non-erosive type (NERD). Mucosal breaks are common endoscopic findings in ERD but not in NERD. According to Nakamura et al. [6], endoscopic findings of NERD are classified into endoscopically normal esophagus (i.e., no specific lesion) and minimal change esophagitis (MCE, non-erosive lesion). The Japanese Study Group for Esophageal Disorders recommends the use of a modified Los Angeles classification wherein the M grade includes erythema indistinguishable from the surrounding area and findings of white turbid discoloration [7]. However, MCE is excluded from the Los Angeles classification because of disagreement among endoscopists [8]. Nonetheless, there are, in part, histologic similarities between MCE and GERD, and some findings are highly consistent among experts, with several positive reports on the association between symptoms and response to medications [9].
Esophageal dysmotility is regarded as one of the major factors in the pathogenesis of GERD [10]. Specifically, Kudara et al. [10] indicated that transient lower esophageal sphincter relaxations, hypotensive lower esophageal sphincter, ineffective esophageal peristalsis, and bolus transit abnormalities were the main factors associated with the development of GERD. Recent studies have also reported that the incidence of such abnormalities increases with the worsening of the reflux disease [11].
In the stomach, the interstitial cells of Cajal generate a slow-wave potential that periodically gives rise to spike potentials, which cause smooth muscle contraction [12]. Electrogastrography (EGG) can record the slow waves and has therefore been used for the diagnosis of gastric dysmotilities [13]. Patients with GERD usually have lower antral motility, decreased slow-wave potential, and delayed gastric emptying time [14], indicating that gastric dysrhythmias contribute to the pathogenesis of GERD [15].
Few studies have compared gastric motility between normal esophagus and MCE, especially in children. Therefore, the purpose of the present study was to investigate the potential differences in gastric motility between endoscopically normal esophagus and MCE in children with reflux symptoms, and to determine whether endoscopic MCE is associated with gastric dysmotilities.
A retrospective study was conducted on 194 children (age <18 years) who underwent both EGG and upper gastrointestinal endoscopy at Gachon University Gil Medical Center between January 2010 and June 2015. Of these, 157 children were included in our study after excluding 37 children for the following reasons: diagnosis of other diseases (eosinophilic esophagitis diagnosed on biopsy, 6 patients; reflux esophagitis with Los Angeles grade A, 5 patients; testicular germ cell tumor, 1 patient; biliary pancreatitis, 1 patient), missing EGG data (16 children), and lack of endoscopic findings (8 children). Of the 157 children included in the study, 153 children underwent tests to detect Helicobacter pylori infection.
MCE was diagnosed using eight endoscopic criteria: erythema, blurring of the Z-line, friability, decreased vascularity, white turbid discoloration, edema and/or accentuation of mucosal folds, and whitish or reddish change [16]. Upper gastrointestinal endoscopy was performed by two endoscopists, and the diagnosis of MCE was made under the consensus of both endoscopists according to the guidelines presented by Kim et al. [16].
Gastric motility was assessed in terms of the following EGG parameters: pre/postprandial dominant frequency, pre/postprandial dominant power, pre/postprandial normogastria, pre/postprandial bradygastria, pre/postprandial tachygastria, pre/postprandial arrhythmia, and power ratio.
The slow waves measured on EGG can be classified as normogastria and arrhythmia waves. Normogastria waves have a frequency of 2–4 cpm (cycles per minute), whereas arrhythmia waves can be subcategorized as bradygastric (dominant peak, 0.5–2.0 cpm), tachygastric (dominant peak, 4.0–9.0 cpm), or arrhythmic (dominant peak, <0.5 or >9.0 cpm). The power ratio, which represents the ratio between the dominant preprandial and postprandial powers, is used as an indicator of changes in gastric contractility. It is generally accepted that a power ratio >1 reflects an increase in gastric contractility after the intervention, whereas a power ratio <1 reflects a decrease in gastric contractility [17].
In all patients, EGG was performed within 5 days before the endoscopic exam. EGG was recorded using a portable EGG recorder (Digitrapper EGG; Synetics Medical, Stockholm, Sweden) after overnight fasting. Medications such as proton pump inhibitors, histamine 2-receptor antagonists, and prokinetics that can affect gastric motor function and acidity were stopped 48 hours prior to the evaluation [18]. Electrodes were placed at three positions: below the left costal margin, between the xyphoid process and the umbilicus, and in the middle of the right upper quadrant. The child was positioned with the upper body at 45° inclination. A preprandial signal was acquired for 15 minutes. Afterwards, the child was instructed to consume the test meal (gimbap; dried seaweed rolls), and a postprandial signal was acquired for another 15 minutes.
The medical records were examined retrospectively to extract demographic characteristics including age, sex, weight, height, and body mass index. The nature and duration of the chief complaint were investigated, including abdominal pain or heartburn, vomiting and/or nausea, dyspepsia, and others. The following symptoms associated with GERD were also investigated: nausea, vomiting, hematemesis, diarrhea, anorexia, wheezing, stridor, cough, weight loss or poor weight gain, and recurrent pneumonia. H. pylori infection was diagnosed when at least one of the following tests was positive: urea-breath test, rapid urease test, stool H. pylori antigen test, or biopsy.
The comparisons between groups employed the chi-square test or Fisher's exact test for categorical variables, while Student's t-test or the Mann-Whitney U-test were used for the comparison of continuous variables. Logistic regression analysis was used to estimate the correlation between each endoscopic finding of MCE and each EGG parameter. A p-value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
The age was significantly higher among children with MCE than among those with normal esophagus (13.46±3.08 vs. 11.11±3.46 years, p=0.007). The Z-score was utilized to compare the groups in terms of weight, height, and body mass index, which could be affected by sex and age, and no significant between-group difference was found. Similarly, there were no significant between-group differences in chief complaint, symptoms and signs, or duration of symptoms (Table 1).
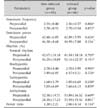
Only the power ratio differed between children with normal esophagus and those with MCE (p=0.021), whereas other EGG parameters such as pre- and postprandial dominant frequency, dominant power, and percent of pre- or postprandial normogastria, tachygastria, or bradygastria did not differ between the groups (Table 2).
Among children with MCE, a significant association was identified between the finding of erythema and power ratio (p=0.049), between the finding of friability and postprandial dominant frequency (p=0.032), and between the findings of edema and/or accentuation of mucosal folds and preprandial frequency (p=0.006). There was no significant association between other findings of MCE (blurring of the Z-line, decreased vascularity, white turbid discoloration) and other EGG parameters (preprandial dominant frequency; postprandial dominant frequency and power; normogastria, tachygastria, and bradygastria percentages of the preprandial and postprandial rhythm) (Table 3).
Only the association between H. pylori infection and postprandial arrhythmias was identified as significant (normal esophagus, 28.10±17.23; MCE, 33.59±15.52; p=0.041). There was no significant association between H. pylori infection and other EGG parameters or any endoscopic findings of MCE (Table 4).
In the 121 (87.7%) children with MCE who underwent biopsy, the biopsy findings revealed normal esophagus in 58 cases (42.0%), chronic inflammation in 57 cases (41.3%), and congestion in 6 cases (4.3%). There were no significant differences in EGG parameters between children with normal esophagus and those with chronic inflammation (data not shown).
In the present study, we found that the power ratio, which is one of the EGG parameters analyzed, was significantly different between children with normal esophagus and those with MCE, and that some EGG parameters correlated with certain endoscopic findings of MCE. These results are in agreement with previous observations that gastric dysmotility is a principal factor in the pathophysiology of GERD [11].
There have been controversies about the diagnostic capabilities of EGG because of its noninvasive nature and the dependence of EGG parameters on antrum-skin distance [19]. However, Shimada et al. [19] reported that power ratio was independent of antrum-skin distance. In our study, we found significant differences in power ratio recorded on EGG between children with MCE and those with normal esophagus, suggesting that gastric motor dysfunction is associated with MCE in children. This conclusion is also supported by a previous finding that delayed gastric emptying is associated with abnormal power ratio on EGG [15].
According to previous research, the dominant power, which is another EGG parameter analyzed here, is higher in individuals with GERD than in those with normal esophagus [20]. However, in our study, there was no difference in dominant power between the two groups (MCE vs. normal esophagus). This inconsistency may originate from several sources. First, GERD patients have pronounced endoscopic mucosal break, and have more evident symptoms. In contrast, patients with MCE have non-erosive endoscopic lesions and rarely show symptoms. Second, the reliability of EGG parameters ought to be considered. As mentioned above, the diagnostic capability of EGG parameters has been controversial because of their dependence on the antrum-skin distance, as well as because of the scarcity of research on this topic [19]. For example, Han et al. [21] reported that there is incomplete correlation between dominant power and gastric activity.
The body of literature currently available suggests that there is little agreement among endoscopists regarding the diagnosis of MCE [8]. However, according to Armstrong et al. [22], diagnostic consensus is higher for erythema than for other findings in MCE. Another report indicated that, on histological examination, multiple papillary vasodilation, which is commonly observed in GERD, is also observed in erythema of MCE [23]. Our present study revealed that the power ratio of erythema was significantly different between children with normal esophagus and those with MCE, suggesting that erythema in MCE may be similar to GERD in terms of pathophysiological characteristics, which represents further evidence supporting the inclusion of erythema findings in the category of GERD.
We also evaluated the correlation between H. pylori infection and EGG parameters. The relationship between H. pylori infection and reflux disease has been under debate. For example, Eren et al. [24] found no correlation between H. pylori infection and reflux disease, whereas Lupu et al. [25] found an inverse relationship between H. pylori infection and GERD. Other studies indicated that H. pylori infection correlates with antral hypomotility in patients with non-ulcer dyspepsia [26]. In our study, there was significant correlation between H. pylori infection and the EGG parameter postprandial arrhythmia (p <.05). However, there was no correlation between H. pylori infection and endoscopic findings of MCE. Therefore, in the context of previous research, our findings suggest that the arrhythmia associated with H. pylori infection could be caused by gastric dysmotility without reflux disease. Nevertheless, a more in-depth study assessing the correlation between EGG parameters and H. pylori infection is needed.
To our knowledge, this was the first investigation to directly assess the correlation between endoscopic findings and EGG parameters, which represents the key strength of the present study. Nevertheless, some limitations of the present study should be noted. First, the group of children with MCE was significantly larger than the group of children with normal esophagus, and the age distribution of the two groups was also significantly different. This limitation is associated with the retrospective, single-center design of our study. Second, we only measured the correlation of each EGG parameter with each endoscopic finding, and did not consider any objective measures of disease severity. Third, as mentioned previously, the exact relevance of each EGG parameter remains unclear. Moreover, we did not assess the correlation between EGG parameters and endoscopic findings in children with normal esophagus. Fourth, although 24-hour esophageal pH monitoring represents a good evaluation for the diagnosis of NERD, we could not perform 24-hour esophageal pH monitoring in this study because of the reluctance of the pediatric patients and their parents. Finally, there was no follow-up evaluation of children with MCE after treatment. Further in-depth studies with prospective design and larger sample size, and covering several age groups, are necessary. Specifically, a study on whether the severity of symptoms, recurrence, medication, or other medical conditions and evaluations are reflected in EGG parameters would be helpful.
Despite its limitations, the present study indicated that the EGG parameter power ratio differs significantly between normal esophagus and MCE, and that several EGG parameters correlate with specific endoscopic findings of MCE. We also found that H. pylori infection was associated with increased postprandial arrhythmias, thereby implying that H. pylori infection is associated with gastric dysmotility. These results suggest that gastric dysmotility may have a certain role in the development of MCE in children, and that H. pylori infection may affect gastric myoelectric activity.
Figures and Tables
Table 2
Electrogastrography Parameters in Children with Normal Esophagus or Minimal Change Esophagitis (MCE)

References
1. Vandenplas Y, Rudolph CD, Di Lorenzo C, Hassall E, Liptak G, Mazur L, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009; 49:498–547.

2. Onyeador N, Paul SP, Sandhu BK. Paediatric gastroesophageal reflux clinical practice guidelines. Arch Dis Child Educ Pract Ed. 2014; 99:190–193.

3. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005; 54:710–717.

4. Okimoto E, Ishimura N, Morito Y, Mikami H, Shimura S, Uno G, et al. Prevalence of gastroesophageal reflux disease in children, adults, and elderly in the same community. J Gastroenterol Hepatol. 2015; 30:1140–1146.

5. Kwon HJ, Yi DY, Ryoo E, Cho KH, Son DW, Tcha H. Prevalence and risk factors associated with esophagitis in children with abdominal pain. Korean J Pediatr Gastroenterol Nutr. 2008; 11:103–109.

6. Nakamura T, Shirakawa K, Masuyama H, Sugaya H, Hiraishi H, Terano A. Minimal change oesophagitis: a disease with characteristic differences to erosive oesophagitis. Aliment Pharmacol Ther. 2005; 21:Suppl 2. 19–26.

7. Hoshihara Y. Endoscopic findings of GERD. Nihon Rinsho. 2004; 62:1459–1464.
8. Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, et al. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999; 45:172–180.

10. Kudara N, Chiba T, Suzuki K. Gastric emptying and electrogastrography in reflux esophagitis: results in patients showing endoscopically erosive esophagitis under proton pump inhibitor therapy. Hepatogastroenterology. 2010; 57:772–776.
11. Martinucci I, de Bortoli N, Giacchino M, Bodini G, Marabotto E, Marchi S, et al. Esophageal motility abnormalities in gastroesophageal reflux disease. World J Gastrointest Pharmacol Ther. 2014; 5:86–96.

12. Chang FY. Electrogastrography: basic knowledge, recording, processing and its clinical applications. J Gastroenterol Hepatol. 2005; 20:502–516.

13. Jamer T. [Multichannel electrogastrography in pediatrics-progress in standardisation and clinical application]. Dev Period Med. 2014; 18:367–373. Polish.
14. Kamiya T, Adachi H, Hirako M, Shikano M, Matsuhisa E, Wada T, et al. Impaired gastric motility and its relationship to reflux symptoms in patients with nonerosive gastroesophageal reflux disease. J Gastroenterol. 2009; 44:183–189.

15. Cucchiara S, Salvia G, Borrelli O, Ciccimarra E, Az-Zeqeh N, Rapagiolo S, et al. Gastric electrical dysrhythmias and delayed gastric emptying in gastroesophageal reflux disease. Am J Gastroenterol. 1997; 92:1103–1108.
16. Kim JH, Park H, Lee YC. Is minimal change esophagitis really part of the spectrum of endoscopic findings of gastroesophageal reflux disease? A prospective, multicenter study. Endoscopy. 2011; 43:190–195.

17. Yin J, Chen JD. Electrogastrography: methodology, validation and applications. J Neurogastroenterol Motil. 2013; 19:5–17.

18. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008; 10:528–534.

19. Shimada Y, Watanabe M, Shibahara N, Kita T, Itoh T, Terasawa K. Electrogastrographic power ratio in humans is not related to changes in antrum-skin distance but to antral motility. J Gastroenterol. 1998; 33:310–317.

20. Chang FY, Lu CL, Chen CY, Luo JC, Jiun KL, Lee SD, et al. Stomach myoelectrical response of patients with gastroesophageal reflux disease receiving omeprazole treatment. J Gastroenterol Hepatol. 2003; 18:1399–1406.

21. Han SY, Yoon SH, Kim JS, Rhyu BH, Rhyu KW. Incomplete relationship between dominant power of electrogastrography and gastric myoelectrical activity in patients with functional dyspepsia. Korean J Orient Med. 2003; 24:92–101.
22. Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996; 111:85–92.

23. Takubo K, Honma N, Aryal G, Sawabe M, Arai T, Tanaka Y, et al. Is there a set of histologic changes that are invariably reflux associated? Arch Pathol Lab Med. 2005; 129:159–163.

24. Eren M, Çolak Ö, Işksoy S, Yavuz A. Effect of H. pylori infection on gastrin, ghrelin, motilin, and gastroesophageal reflux. Turk J Gastroenterol. 2015; 26:367–372.

25. Lupu VV, Ignat A, Ciubotariu G, Ciubară A, Moscalu M, Burlea M. Helicobacter pylori infection and gastroesophageal reflux in children. Dis Esophagus. 2016; 29:1007–1012.

26. Thor P, Lorens K, Tabor S, Herman R, Konturek JW, Konturek SJ. Dysfunction in gastric myoelectric and motor activity in Helicobacter pylori positive gastritis patients with non-ulcer dyspesia. J Physiol Pharmacol. 1996; 47:469–476.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download