Abstract
Pediatric patients require specialized attention and have diverse demands for proper growth and development, and thus need a different approach and interest in nutritional assessment and supply. Enteral nutrition is the most basic and important method of nutritional intervention, and its indications should be identified. Also, the sites, modes, types, and timing of nutritional intervention according to the patient's condition should be determined. In addition, various complications associated with enteral nutrition supply should be identified, and prevention and treatment are required. This approach to enteral nutrition and proper administration can help in the proper growth and recovery of pediatric patients with nutritional imbalances or nutritional needs.
At any age, the most important aspects in relation to diseases are proper diagnosis and treatment; for this reason, numerous drugs and surgical methods have been developed. In recent years, however, the importance of professional treatment beyond simple medical treatment is increasing, and proper nutritional support is essential [12]. Thus, interest in nutrition is increasing in modern medicine. In the management of complications of diseases or sequelae during and after treatment, the importance of nutritional therapy or artificial nutrition is increasing [345].
Pediatric patients, in particular, require specialized attention and have diverse demands for proper growth and development, not necessarily because of illness [678]. Unlike adults, ongoing care for proper growth is also needed, and the quality of life of parents and other families caring for their children should also be considered. In addition, infants, children, and young adolescents require different medical and psychological approaches because of diseases and prognosis depending on age and weight [678].
Therefore, for nutritional approach and artificial nutrition, gastrointestinal function such as digestion and absorption is important, as well as age and clinical condition. In addition, considerations should be given to the likelihood of oral intake, the degree of functioning, the possibility of intervention, the cost of nutritional management, diet, and compliance [26]. In this article, we will examine the basic concepts of nutritional therapy in children, particularly enteral nutrition (EN).
The initial steps to nutritional therapies and approaches are to determine nutritional status through nutritional counseling [12]. Whether an intervention such as artificial nutrition is necessary in consideration of disease state, economic power, and ethical issues is determined. Then, oral nutritional supplements are added, and EN or parenteral nutrition (PN) according to the indications are considered. Essentially, EN is the basic artificial nutrition performed by medical doctors [2].
Nutritional support is provided to pediatric patients under two possible conditions [67]. The first is to supply <60% to 80% of the nutritional requirements for >10 days, or >4 to 6 hours per day of total feeding time for children with disabilities. In the case of insufficient oral intake, nutritional support should be initiated within 5 days at the age of 1 year and within 3 days at the age of <1 year.
The second is wasting and stunting status as follows: 1) inadequate growth and >1 month of weight loss for <2 years; 2) weight loss of ≥3 months or no weight gain at the age of 2 years; 3) >2 stages of weight change in the growth charts; 4) triceps skinfolds consistently below the fifth percentile of age; 5) decreased height velocity by ≥0.3 SD per year; and 6) decreased height velocity by >2 cm per year during puberty.
The concept of EN is known to have originated from ancient Egypt and later Greece [91011]. Initially, the practice was to put food into the rectum. In the 16th century, a hollow tube with a bladder attached is inserted into the esophagus. In the 18th century, orogastric tubes were designed to supply jellies and eggs along with milk and water. By the 19th century, basic foods such as broths, eggs, milk, and even alcohol were fed through rudimentary tubes to the esophagus. In the 1930s, protein hydrolysate formulations were supplied to surgical patients, and in the 1940s, the first infant formula was developed. Since the 1980s, EN has been recognized as a safe, effective, and cost-effective way to provide adequate nutrition. Accordingly, various devices for nutritional supply have been developed, and formulas with various indications and purposes have been produced [1112].
In the past, EN has been traditionally defined as providing food to the stomach or small intestine beyond the esophagus through a tube. However, recently, the need for nutritional intervention has increased; thus, various oral nutritional supplements have been developed. EN is defined as the use of oral food for medical purposes [2613]. EN is easier and safer to supply than PN. As intravenous access is not needed, EN has no side effects such as catheter-related or metabolic complications, and is advantageous in preserving the gastrointestinal function [1415161718].
As pediatric patients differ in the type and course of nutrition depending on age, consideration should be given to the EN supply [619]. The current age, life expectancy, acute or chronic disease, adverse effect of treatment, or temporary deterioration due to disease should be determined. The method and duration of EN should be decided in accordance with whether the patient's condition is stable or reversible, and with the function of the gastrointestinal tract [2021].
The indications for EN in pediatric patients are not significantly different from those required in nutritional support (Table 1). EN is needed if the general dietary intake alone cannot meet the energy and nutritional needs of children with growth retardation, weight faltering, or weight deficit [11]. It can also be considered useful for the treatment of diseases such as Crohn's disease, food allergy, and intolerance [2223]. However, the most important condition is that the function of the gastrointestinal tract allows for EN, at least in part [6]. It may also be used for various diagnoses or illnesses, but its use should be determined by consideration of the cost of the patient, or whether it is feasible or not [2425]. In neonates, EN supply is required depending on the condition in situations such as premature or necrotizing enterocolitis [7].
Absolute contraindications to EN are problems with the gastrointestinal function, such as paralytic or mechanical ileus, and intestinal obstruction or perforation. Relative contraindications include intestinal dysmotility, necrotizing enterocolitis, toxic megacolon, diffuse peritonitis, gastrointestinal bleeding, and high-output enteric fistula. However, if possible, full fasting should be avoided and a minimum intestinal nutritional supply should be maintained [2627].
The decision on the location and route of EN administration is based on the patient's disease status, the structural and functional status of the gastrointestinal tract, the purpose and duration of EN, and the risk of aspiration. The mode of delivery of nutrients is preferably through the stomach because it is more physiological [611].
Gastric feeding has the risk of gastroesophageal reflux and pulmonary aspiration. However, it can play a bactericidal role through gastric hydrochloric acid and helps to absorb certain nutrients. Bolus feeding is also possible because the position is fixed and easy to administer, and the stomach serves as a reservoir [36]. This intermittent bolus feeding provides a cyclic surge of gastrointestinal hormones, so it has a trophic effect on the intestinal mucosa, allows the feeding patient to freely perform activities, and is more physiological [628]. It does not require a feeding pump and is cheap. However, it carries a risk of osmotic diarrhea and is disabled in jejunal feeding (Table 2).
Post-pyloric feeding is performed in situations where gastric feeding is difficult, such as tracheal aspiration, gastroparesis, gastric outlet dysfunction, or previous gastric surgery. Rapid infusion of nutrients is not possible, so intermittent or continuous infusion should be performed. However, this carries the risk of high-energy, hyperosmolar feeding. Therefore, post-pyloric feeding in preterm infants requires more attention and carries a higher risk of complications. Nevertheless, continuous feeding delivered through infusion at a constant rate provides constant mucosal stimulation to aid intestinal adaptation and enable optimal absorption. It also has a lower probability of emesis than intermittent feeding, and is more effective at enteral balance and weight gain [2930]. However, the development of taste or oral motor function may become problematic (Table 2).
To compensate for the advantages and disadvantages of these feeding methods, a method of preserving the oral feeding skill through the combination of bolus feeding during the day and continuous feeding during the night may be used depending on the patient's condition.
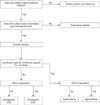
To determine the route of administration for EN delivery, the expected duration of EN and the underlying disease of the patient or the structure and functioning of the gastrointestinal tract should be considered [6731]. Technical experience or cost, psychological approaches, or patient activity may also be an additional consideration (Fig. 1).
If a short period of EN of <4 weeks is required, nutrition should be directly supplied to the stomach, duodenum, or jejunum via a tube. Gastric tube feeding, or inserting a feeding tube directly through the mouth or nose, is a relatively less invasive procedure and less difficult to locate. It is the most common and effective method of short-term EN supply, but the possibility of blockage is high because of the small luminal diameter. Nasoduodenal or nasojejunal tube feeding can be performed when gastric tube feeding is difficult owing to the risk of aspiration. Owing to the difficulty of positioning, various methods are used, such as endoscopy, fluoroscopy, or administration of prokinetic drugs [32333435].
Even if EN is required for >4 weeks, it is positioned according to the risk of aspiration. Nutrition is supplied endoscopically or through a surgical access established using gastrostomy or jejunostomy [31]. Chronic diseases associated with nutritional imbalance or neurological abnormalities such as cerebral palsy, neuromuscular disorder, and coma are indications for percutaneous endoscopic gastrostomy (PEG) or jejunostomy (PEJ). PEG(J) is also considered for feeding and decompression if malignant tumors of the head, neck, or esophagus or chronic intestinal pseudo-obstruction is present [36]. PEG(J) feeding has fewer complications and discomforts, such as irritation, ulceration, bleeding, displacement, and clogging, than nasogastric tube feeding [37]. However, it may be difficult to apply according to the abdominal wall or cooperative condition of the patient, and may be a contraindication even if life expectancy is limited or serious coagulation disorders are present [3138].
Endoscopic techniques are the most commonly used method for enteral access, but laparoscopic, sonographic, fluoroscopic, and surgical methods are also often used [31]. In the case of the PEG tube, it can be used for a long time in a fixed position, is easy to use and remove, and is associated with relatively fewer complications. The button- or balloon-type PEG tube is designed to be fixed in the stomach and considered more frequently for pediatric patients of relatively older age. PEJ is similar to PEG but forms a tract between the duodenum and the abdominal wall. After laboratory tests (hemoglobin, platelet, and coagulation tests) are performed before and after the PEG or PEJ procedure for checking for contraindications, the procedure is performed under aseptic conditions after prophylactic antibiotic treatment [31]. After the procedure, the patient can start diet 6 hours later and full feeding after 24 hours.
Mechanical complications are common, but in most cases, they are not as serious as central catheter-related complications [11]. The naso-enteral tube may cause a problem of clogging or missing the tube itself, which may cause discomfort to the patient [6]. Caution is needed because it may cause perforation or related complications. Gastrostomy and enterostomy tubes may also cause similar complications and local irritation. Stoma-related complications may result in an enlarged stoma site due to a large wall incision, leakage of nutrients or gastric juice, and enterocutaneous fistula after removal [6].
Infectious complications can occur in two major directions. First, wound infection such as purulent discharge, cellulitis, and peristomal abscess or local and systemic septicemia associated with feeding devices can occur [39404142]. Prevention and treatment through antibiotics and dressings in sterile conditions are important before and after the procedure. The following infectious complications are contamination of formulas and delivery sets [43]. The cause of bacterial contamination is not known precisely, but often sepsis may occur, and up to 35% to 50% of cases have been reported in pediatric hospitals [44]. Coagulase-negative staphylococci, streptococci, and gram-negative bacilli cause infections [6]. Formulas should always be prepared in a sanitary environment, as infection can occur due to insufficient hand washing or lack of awareness of hygiene, and repeated use of food storage containers. Reducing exposure time to contamination, such as through feeding hang time or minimizing time to exposure after opening the formula, may also be effective for infection control [45].
Gastrointestinal complications include abdominal discomfort, bloating, and cramping [11]. Excessive infusion rate, slow gastric emptying, constipation, and psychological factors can cause nausea and vomiting, and dislodged tubes and intolerance of bolus feeds can lead to regurgitation or aspiration [6]. Diarrhea may also occur due to dietary intake that is incompatible with gastrointestinal function, intolerance of bolus fees, excessive infusion rate, high feed osmolarity, and microbial contamination [6].
Although metabolic complications are not common occurrences in EN, patients with chronic nutritional imbalance or cardiac, liver, or renal problems require more attention. Careful attention should be paid to the possibility of refeeding syndrome during abrupt feeding of high-energy nutrition in patients with chronic nutritional imbalances [46]. When excessive amounts of carbohydrates are supplied, phosphorus, magnesium, and potassium move into the cells due to sudden increase in insulin secretion [6]. As hypophosphatemia can lead to heart failure, arrhythmia, and death, the initial supply volume or calories should be at <75% of the requirement.
Unexpected interactions may occur when drugs are administered via enteral feeding tubes [611]. Thus, before administration of the drug through the intestinal tract, other possible routes should be considered and the administration of coated or slowly degrading drugs through the tube should be avoided. If the tube is the only route for drug administration, drugs should be administered in portions; pills should be mixed with water and gelatin capsules should be dissolved in warm water before administration.
EN is the preferred method of nutritional supplementation if sufficient calories are not available in oral feeding. It is preferred to nourish the stomach from above it rather than supplying nutrients to the duodenum beyond the pylorus. Intermittent bolus feeding is also preferred because it is more physiological than continuous feeding in small amounts. Supply through gastrostomy or enterostomy should be considered when long-term nutrition of >4 weeks is expected. Complications should be minimized through careful attention and regular monitoring, as problems such as EN-related infections or metabolic abnormalities can occur.
Figures and Tables
References
1. Druml C, Ballmer PE, Druml W, Oehmichen F, Shenkin A, Singer P, et al. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr. 2016; 35:545–556.

2. Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 2017; 36:49–64.

3. American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. Clinical guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients, 2009. JPEN J Parenter Enteral Nutr. 2009; 33:255–259.
4. Jonkers CF, Prins F, Van Kempen A, Tepaske R, Sauerwein HP. Towards implementation of optimum nutrition and better clinical nutrition support. Clin Nutr. 2001; 20:361–366.

5. Planas M, Camilo ME. Artificial nutrition: dilemmas in decision-making. Clin Nutr. 2002; 21:355–361.

6. Braegger C, Decsi T, Dias JA, Hartman C, Kolacek S, Koletzko B, et al. ESPGHAN Committee on Nutrition. Practical approach to paediatric enteral nutrition: a comment by the ESPGHAN committee on nutrition. J Pediatr Gastroenterol Nutr. 2010; 51:110–122.

7. Axelrod D, Kazmerski K, Iyer K. Pediatric enteral nutrition. JPEN J Parenter Enteral Nutr. 2006; 30:1 Suppl. S21–S26.

8. Agostoni C, Decsi T, Fewtrell M, Goulet O, Kolacek S, Koletzko B, et al. ESPGHAN Committee on Nutrition. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008; 46:99–110.

9. Harkness L. The history of enteral nutrition therapy: from raw eggs and nasal tubes to purified amino acids and early postoperative jejunal delivery. J Am Diet Assoc. 2002; 102:399–404.
10. Chernoff R. An overview of tube feeding: from ancient times to the future. Nutr Clin Pract. 2006; 21:408–410.

11. McCallum Z, Bines JE. Enteral Nutrition and Formulas. In : Duggan C, Watkins JB, Koletzko B, Walker WA, editors. Nutrition in pediatrics. 5th ed. Shelton, CT: PMPHUSA;2016. p. 1023–1034.
12. Phillips MS, Ponsky JL. Overview of enteral and parenteral feeding access techniques: principles and practice. Surg Clin North Am. 2011; 91:897–911.

13. Valentini L, Volkert D, Schütz T, Ockenga J, Pirlich M, Druml W, et al. Suggestions for terminology in clinical nutrition. e-SPEN. 2014; 9:e97–108.

14. Gramlich L, Kichian K, Pinilla J, Rodych NJ, Dhaliwal R, Heyland DK. Does enteral nutrition compared to parenteral nutrition result in better outcomes in critically ill adult patients? A systematic review of the literature. Nutrition. 2004; 20:843–848.

15. Shojania KG, Duncan BW, McDonald KM, Wachter RM, Markowitz AJ. Making health care safer: a critical analysis of patient safety practices. Evid Rep Technol Assess (Summ). 2001; (43):i–x.
16. Wildhaber BE, Yang H, Spencer AU, Drongowski RA, Teitelbaum DH. Lack of enteral nutrition--effects on the intestinal immune system. J Surg Res. 2005; 123:8–16.

17. Kudsk KA. Effect of route and type of nutrition on intestine-derived inflammatory responses. Am J Surg. 2003; 185:16–21.

18. Reddy P, Malone M. Cost and outcome analysis of home parenteral and enteral nutrition. JPEN J Parenter Enteral Nutr. 1998; 22:302–310.

19. Kleinman RE. Enteral nutrition support. In : Kleinman RE, editor. Pediatric nutrition handbook. Elk Grove Village, IL: American Academy of Pediatrics;2004. p. 280–290.
20. Avitzur Y, Courtney-Martin G. Enteral approaches in malabsorption. Best Pract Res Clin Gastroenterol. 2016; 30:295–307.

21. Goulet O, Ruemmele F, Lacaille F, Colomb V. Irreversible intestinal failure. J Pediatr Gastroenterol Nutr. 2004; 38:250–269.

22. Critch J, Day AS, Otley A, King-Moore C, Teitelbaum JE, Shashidhar H. NASPGHAN IBD Committee. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012; 54:298–305.

23. Hays T. Special considerations for managing food allergies. JPEN J Parenter Enteral Nutr. 2012; 36:1 Suppl. 56S–59S.

24. Heyman MB, Harmatz P, Acree M, Wilson L, Moskowitz JT, Ferrando S, et al. Economic and psychologic costs for maternal caregivers of gastrostomy-dependent children. J Pediatr. 2004; 145:511–516.

25. de Lucas C, Moreno M, López-Herce J, Ruiz F, Pérez-Palencia M, Carrillo A. Transpyloric enteral nutrition reduces the complication rate and cost in the critically ill child. J Pediatr Gastroenterol Nutr. 2000; 30:175–180.

26. Ohta K, Omura K, Hirano K, Kanehira E, Ishikawa N, Kato Y, et al. The effects of an additive small amount of a low residual diet against total parenteral nutritioninduced gut mucosal barrier. Am J Surg. 2003; 185:79–85.

27. Tyson JE, Kennedy KA. Minimal enteral nutrition for promoting feeding tolerance and preventing morbidity in parenterally fed infants. Cochrane Database Syst Rev. 2000; (2):CD000504.
28. Aynsley-Green A, Adrian TE, Bloom SR. Feeding and the development of enteroinsular hormone secretion in the preterm infant: effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatr Scand. 1982; 71:379–383.

29. Horn D, Chaboyer W, Schluter PJ.. Gastric residual volumes in critically ill paediatric patients: a comparison of feeding regimens. Aust Crit Care. 2004; 17:98–100.

30. Serpa LF, Kimura M, Faintuch J, Ceconello I. Effects of continuous versus bolus infusion of enteral nutrition in critical patients. Rev Hosp Clin Fac Med Sao Paulo. 2003; 58:9–14.

31. Löser C, Aschl G, Hébuterne X, Mathus-Vliegen EM, Muscaritoli M, Niv Y, et al. ESPEN guidelines on artificial enteral nutrition--percutaneous endoscopic gastrostomy (PEG). Clin Nutr. 2005; 24:848–861.
32. Brandt CP, Mittendorf EA. Endoscopic placement of nasojejunal feeding tubes in ICU patients. Surg Endosc. 1999; 13:1211–1214.

33. Heiselman DE, Hofer T, Vidovich RR. Enteral feeding tube placement success with intravenous metoclopramide administration in ICU patients. Chest. 1995; 107:1686–1688.

34. Gharpure V, Meert KL, Sarnaik AP. Efficacy of erythromycin for postpyloric placement of feeding tubes in critically ill children: a randomized, double-blind, placebo controlled study. JPEN J Parenter Enteral Nutr. 2001; 25:160–165.

35. Huerta G, Puri VK. Nasoenteric feeding tubes in critically ill patients (fluoroscopy versus blind). Nutrition. 2000; 16:264–267.

36. Mekhail TM, Adelstein DJ, Rybicki LA, Larto MA, Saxton JP, Lavertu P. Enteral nutrition during the treatment of head and neck carcinoma: is a percutaneous endoscopic gastrostomy tube preferable to a nasogastric tube? Cancer. 2001; 91:1785–1790.

37. Norton B, Homer-Ward M, Donnelly MT, Long RG, Holmes GK. A randomised prospective comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding after acute dysphagic stroke. BMJ. 1996; 312:13–16.

38. American Society for Gastrointestinal Endoscopy. Role of PEG/PEJ in enteral feeding. Gastrointest Endosc. 1998; 48:699–701.
39. Dautle MP, Wilkinson TR, Gauderer MW. Isolation and identification of biofilm microorganisms from silicone gastrostomy devices. J Pediatr Surg. 2003; 38:216–220.

40. Ségal D, Michaud L, Guimber D, Ganga-Zandzou PS, Turck D, Gottrand F. Late-onset complications of percutaneous endoscopic gastrostomy in children. J Pediatr Gastroenterol Nutr. 2001; 33:495–500.

41. Kobak GE, McClenathan DT, Schurman SJ. Complications of removing percutaneous endoscopic gastrostomy tubes in children. J Pediatr Gastroenterol Nutr. 2000; 30:404–407.

42. Avitsland TL, Kristensen C, Emblem R, Veenstra M, Mala T, Bjørnland K. Percutaneous endoscopic gastrostomy in children: a safe technique with major symptom relief and high parental satisfaction. J Pediatr Gastroenterol Nutr. 2006; 43:624–628.

43. Bott L, Husson MO, Guimber D, Michaud L, Arnaud-Battandier F, Turck D, et al. Contamination of gastrostomy feeding systems in children in a home-based enteral nutrition program. J Pediatr Gastroenterol Nutr. 2001; 33:266–270.

44. Roy S, Rigal M, Doit C, Fontan JE, Machinot S, Bingen E, et al. Bacterial contamination of enteral nutrition in a paediatric hospital. J Hosp Infect. 2005; 59:311–316.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download