Abstract
Purpose
Our aim in this study is to investigate efficacy of topical lidocaine spray for sedated esophagogastroduodenoscopy (EGD) in children.
Methods
The endoscopy of children aged between 3-18 years who underwent EGD in our endoscopy unit. Intravenous (IV) midazolam and ketamine were used for sedation. Prior to sedation, endoscopy nurse applied topical lidocaine 10% with pump spray at 1 mg/kg dose in group 1, and distilled water via identically scaled pump spray in group 2, in a double blinded fashion.
Results
Sedation was not applied in 24.1% of the cases in topical lidocaine spray group (LS group) and in 5.7% of the cases in distilled water spray group (DS group). Gag reflex was observed in 6.5% of cases in LS group and 33.3% of cases in DS group (p=0.024), increased oral secretion was observed in 9.3% of cases in LS group and 51.7% of cases in DS group (p=0.038), sore throat was observed in 3.7% of cases in LS group and 35.6% of cases in DS group (p=0.019) and the difference was statistically significant.
Esophagogastroduodenoscopy (EGD) is an essential and very commonly used procedure for the evaluation of a multitude of gastrointestinal (GI) symptoms including abdominal pain, hemorrhage, dysphagia, odynophagia, and reflux [12]. To increase patient tolerance, EGD is generally performed using topical pharyngeal anesthesia and/or sedation [34]. Traditionally, topical lidocaine spray is used in combination with intravenous (IV) analgesics and sedatives in EGD, and increases success rate of endoscopy [5]. Several studies have found the lidocaine pharyngeal anesthesia to be beneficial [67]. Additionally, topical lidocaine suppresses gag reflex which is frequently observed during EGD, and therefore increases both patient's and endoscopist's satisfaction [68]. Topical lidocaine spray is often used during endoscopic procedures performed in adults; but has a limited use in pediatric practice. The aim of our study is to investigate efficacy of topical lidocaine spray for sedated EGD in children.
The endoscopy of children aged between 3–18 years who underwent EGD in Akdeniz University Pediatric Gastroenterology Endoscopy Unit between August 2015 to February 2016. The study was approved by Akdeniz University Clinical Research Ethics Committee (Date: 12/08/2015, Issue No: 24). The verbal and written consents of the families were obtained before the EGD procedure.
Patients aged between 3-18 years who underwent EGD due to dyspepsia, chronic diarrhea, suspected celiac disease or other reasons (varices, corrosive esophagitis, balloon dilation) were included in the study.
Patients with cardiac, pulmonary, neurological or metabolic diseases, or hepatic failure, or who are known to have hypersensitivity to midazolam, ketamine and/or topical lidocaine spray excluded from the study.
Randomization was ensured by the random application of sprays with unknown content and just a code number on them on the patients by an endoscopy nurse.
It was ensured by using sprays with unknown content with the same color, smell and taste and with just a code number on them.
The study was conducted on class 1 and class 2 patients according to the classification of American Society of Anesthesiologists [9].
In cases, slow IV infusion of midazolam (Dormicum; Roche, İstanbul, Turkey) at 0.1 mg/kg (maximum 4 mg) dose was followed by IV ketamine (Ketalar; Pfizer, Sandwich, UK) at 0.5 mg/kg (maximum 2 mg/kg) dose administered. Sedation with the doses specified above was accepted as normal dose, whereas in case a smaller dose was sufficient for sedation, it was accepted as low-dose. Prior to sedation, endoscopy nurse as random applied topical lidocaine 10 % with pump spray at 1 mg/kg dose (Xylocaine; AstraZeneca, Silk Road, UK) and distilled water with mint oil via identically scaled pump spray in a double-blind. EGD procedure was performed by pediatric endoscopist, using EG530 WR (diameter 9.4 mm; Fujinon, Tokyo, Japan). Effectiveness of sedation was assessed by the endoscopist using modified Ramsay sedation scale (RSS) [10]. Scoring in RSS scale is ranked in 6 points based on patient's reaction and cooperation. According RSS, R5 (deep sedation, patient only reacts to painful stimulus) was accepted as contraindication for additional doses of midazolam.
Patients were monitored throughout the procedure. Endoscopy nurse recorded cardiac apex beat, peripheral oxygen saturation and arterial blood pressure. All patients received oxygen at 2 L/min rate during the procedure via nasal cannula. As side effects, emergent situations such as hypoxia (peripheral oxygen saturation <90%), tachycardia (increase in cardiac apex beat that is above 30% of age-normal), bradycardia (decrease in cardiac apex beat that is above 30% of age-normal), hypertension (increase in blood pressure that is above 20% of age-normal), hypotension (decrease in blood pressure that is above 20% of age-normal), gag reflex, increased oral secretion, flushing, urticaria, vomiting, apnea, convulsion and oxygen requirement with mask were noted (peripheral oxygen saturation <90% was applied).
Besides complications specified above that occurred during the procedure, euphoria, dysphoria, sore throat, vertigo, visual problems like double vision and nystagmus, and emergent situations like arrhythmia, convulsion, hallucination, and other situations that occurred after the procedure were recorded [11].
Recovery time was evaluated according to respiration, energy, alertness, circulation, and temperature (REACT) scale [12]. This scale is scored between 0 and 10, and is based on disease activity, body temperature, cognitive state, circulatory and pulmonary condition. Patients whose REACT score is 10 can be discharged from the endoscopy unit. Complications that existed at the time of patient's discharge were recorded.
The primary outcome measures indicate the efficacy of topical lidocaine in sedated children who have undergone an EGD procedure.
Secondary outcome measures include the reduction of side effects that occur due to IV midazolam and ketamine, such as apnea, hypoxia, vomiting, agitation and allergic reactions, with the use of topical lidocaine.
The statistical analysis was conducted with SPSS 15.0 (SPSS Inc., Chicago, IL, USA) software program and MS Office Excell ver. 2010 were used for the statistical analysis of the data. Normality assessment of the data was made with One-Sample Kolmogorov-Smirnov test. Comparison of the data was made with Mann-Whitney U test, chi-square test and Fischer's exact test. For descriptive statistics, numerical variables were expressed as mean±standard deviation, and categorical data were expressed as count and percentage. Statistical level of significance was accepted as p<0.05.
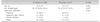
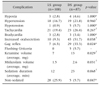
In our study, totally 287 patients who underwent upper GIS endoscopy in our endoscopy unit between August 2015 and February 2016. Twenty-three patients did not volunteer for the study, endoscopy procedure could not be completed in 7 patients excluded (because the patients woke up during EGD, the procedure was terminated), 59 patients had cardiac, pulmonary, neurological, metabolic disease or hepatic failure, and 3 patients had known allergy for ketamine, midazolam, and/or lidocaine; therefore these patients were not included in the study. Because the remaining 195 patients received topical lidocaine spray group (LS group) and distilled water spray group (DS group) consecutively, they were randomly categorized to LS and DS groups. Mean age in the study group was 12.9±3.6 years, and 57.4% of cases were female and 42.6% were male in this group. According to upper endoscopy indications, endoscopy was performed due to dyspepsia in 65%, suspected celiac disease in 13%, chronic diarrhea in 4% and other reasons (evaluation of varices, corrosive esophagitis, balloon dilatation) in 17% of the cases. Comparison of patients in LS and DS groups for age, sex and indication did not show a statistically significant difference (Table 1). Twenty-six of 108 patients (24.1%) in LS group did not receive sedation, whereas 5 of 87 patients (5.7%) in DS group did not receive sedation. The remaining 82 patients in each group received sedation. Fig. 1 shows patient flow diagram. Regarding the patients who received sedation, 27% in LS group and 11% in DS group received low-dose sedation and the difference was statistically significant (p=0.027). According to RSS classification, majority of the patients in both groups were categorized ad R4 or R5. Fig. 2 shows distribution of groups according to RSS and there was no statistical difference between the groups (p=0.982). Regarding complications that developed during EGD, gag reflex was observed in 6.5% of cases in LS group and 33.3% of cases in DS group (p=0.024), increased oral secretion was observed in 9.3% of cases in LS group and 51.7% of cases in DS group (p=0.038) and the difference was statistically significant. Hypertension was observed in 16.7% and 21.8% of cases in LS and DS groups, respectively; whereas tachycardia was observed in 19.4% and 26.4% of cases in LS and DS groups, respectively, and both groups had similar rates for these two signs (p=0.960, p=0.267). Ketamine volume was observed 7 and 13 mg in LS and DS groups (p=0.029), midazolam volume 1.5 and 2.6 mg in LS and DS groups (p=0.031), sedation duration 12 and 23 minute in LS and DS groups (p=0.068) and non-sedated 25.9% and 5.7% in LS and DS groups (p=0.047) respectively, the difference was statistically significant (Table 2).
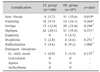
Regarding complications that developed after EGD, sore throat was observed in 3.7% of cases in LS group and 35.6% of cases in DS group (p=0.019) and the difference was statistically significant. Vomiting was observed in 9.3% of cases in LS group and in 16.1% of cases in DS group (p=0.264). Vertigo was observed in 12.0% of cases in LS group and in 23.0% of cases in DS group (p=0.298). Diplopia was observed in 24.1% of cases in LS group and in 35.6% of cases in DS group (p=0.371). Euphoria was not observed in any of the cases in LS group and in only 3 patients (3.5%) in DS group. Dysphoria was observed in similar rates in both groups (2.8% and 4.6%, respectively; p=0.251). Emergent conditions developed in only one case 0.9%) in LS group and in 3 patients (3.5%) in DS group; and the no difference was statistically significant (p=0.137, Table 3).
Topical pharyngeal anesthesia before IV sedation for EGD in children is not a common practice. However, combination of midazolam and ketamine is often used along with topical pharyngeal anesthesia in children for practicability of the procedure. According to a review of literature, topical pharyngeal anesthesia along with IV sedation in EGD seems advantageous [13].
At the same time, topical lidocaine use has been shown to reduce the need for IV sedation. In a study comparing lidocaine gel and lidocaine spray among other studies on this topic, it was observed that there was less need for IV sedation in the lidocaine gel group [14]. Similarly, in a comparison between lidocaine lollipop and lidocaine spray, it was observed that there was less need for IV sedation in the lollipop group [15]. In a similar fashion, our study showed that a lesser extent of IV sedation was applied in the LS group compared to the DS group.
While several studies show that topical lidocaine use reduces the gag reflex [1516]. Our study showed that the gag reflex was observed to a lesser extent in the LS group than the DS group (6–35%, p=0.024, respectively), compatible with the literature.
In studies comparing the increase in oral secretion, it was observed to a lesser extent in the topical pharyngeal lidocaine group [17]. Our data show that there is less increase in oral secretion in the LS group (9.3%) compared to the DS group (51.7%), compatible with the literature (p=0.038). In a study done in our country comparing sore throat, sore throat was observed the least in the lidocaine benzydamine group in the comparison of lidocaine spray, lidocaine benzydamine spray and benzydamine spray, while it was observed the most in the benzydamine group [18]. In our study, sore throat was observed to a significantly lesser degree in the LS group (3.7%) compared to the DS group (35.6%) compatible with the literature (p=0.019).
In another study comparing midazolam-ketamine, midazolam-placebo and midazolam-fentanyl, dizziness, vomiting and cough were most frequently observed in the midazolam-ketamine group and a significant difference was observed between the midazolam-ketamine group and the midazolam-fentanyl group in terms of diplopia and vomiting (p=0.004 and 0.002, respectively) [19].
In another study, there was no difference between ketamine group and ketamine-midazolam group regarding vomiting, and vomiting was observed totally in 17% of cases [20].
Langston et al. [21] observed vomiting in 18.9% of cases in ketamine group. Our results are similar to the studies given above in terms of vomiting rate. We observed vomiting totally in 12.3% of cases, 9.3% in LS group and 16.1% in DS group; and the difference between groups was not statistically significant (p=0.264). Vertigo was observed remarkably lower in LS group (12.0%) than in DS group (23.0%); however, the difference was not statistically significant (p=0.298). Diplopia was observed with similar frequencies in LS and DS groups (24.1% vs. 35.6%, respectively; p=0.371).
In our study, frequency of dysphoria was similar in LS and DS groups and was 2.8% and 4.6%, respectively (p=0.251). Our results are similar to that of Green et al. [22] in which they found this rate 2.4% with ketamine.
Emergent situations were rare in our study, with 0.9% rate in LS group and 3.5% in DS group. Wathen et al. [23] reported emergent situations with 7.1% rate in ketamine group and 6.2% rate in midazolamketamine group.
In conclusion, the study showed that topical pharyngeal lidocaine reduces both requirement and amount of IV sedation before EGD in children and sore throat, gag reflex and decreased oral secretion increase.
Figures and Tables
Fig. 1
Patient flow diagram. LS group: topical lidocaine spray group, DS group: distilled water spray group.

References
1. Clarke GA, Jacobson BC, Hammett RJ, Carr-Locke DL. The indications, utilization and safety of gastrointestinal endoscopy in an extremely elderly patient cohort. Endoscopy. 2001; 33:580–584.

2. Van Kouwen MC, Drenth JP, Verhoeven HM, Bos LP, Engels LG. Upper gastrointestinal endoscopy in patients aged 85 years or more. Results of a feasibility study in a district general hospital. Arch Gerontol Geriatr. 2003; 37:45–50.

3. al-Atrakchi HA. Upper gastrointestinal endoscopy without sedation: a prospective study of 2000 examinations. Gastrointest Endosc. 1989; 35:79–81.

4. Keeffe EB, O'Connor KW. 1989 A/S/G/E survey of endoscopic sedation and monitoring practices. Gastrointest Endosc. 1990; 36:3 Suppl. S13–S18.
5. Davis DE, Jones MP, Kubik CM. Topical pharyngeal anesthesia does not improve upper gastrointestinal endoscopy in conscious sedated patients. Am J Gastroenterol. 1999; 94:1853–1856.

6. Hedenbro JL, Ekelund M, Jansson O, Lindblom A. A randomized, double-blind, placebo-controlled study to evaluate topical anaesthesia of the pharynx in upper gastrointestinal endoscopy. Endoscopy. 1992; 24:585–587.

7. Evans LT, Saberi S, Kim HM, Elta GH, Schoenfeld P. Pharyngeal anesthesia during sedated EGDs: is "the spray" beneficial? A meta-analysis and systematic review. Gastrointest Endosc. 2006; 63:761–766.

8. Gordon MJ, Mayes GR, Meyer GW. Topical lidocaine in preendoscopic medication. Gastroenterology. 1976; 71:564–569.

9. Lowrie L, Weiss AH, Lacombe C. The pediatric sedation unit: a mechanism for pediatric sedation. Pediatrics. 1998; 102:E30.

10. Martinez JL, Sutters KA, Waite S, Davis J, Medina E, Montano N, et al. A comparison of oral diazepam versus midazolam, administered with intravenous meperidine, as premedication to sedation for pediatric endoscopy. J Pediatr Gastroenterol Nutr. 2002; 35:51–58.

11. Green SM, Li J. Ketamine in adults: what emergency physicians need to know about patient selection and emergence reactions. Acad Emerg Med. 2000; 7:278–281.

12. Tolia V, Peters JM, Gilger MA. Sedation for pediatric endoscopic procedures. J Pediatr Gastroenterol Nutr. 2000; 30:477–485.

13. Quine MA, Bell GD, McCloy RF, Charlton JE, Devlin HB, Hopkins A. Prospective audit of upper gastrointestinal endoscopy in two regions of England: safety, staffing, and sedation methods. Gut. 1995; 36:462–467.

14. Soweid AM, Yaghi SR, Jamali FR, Kobeissy AA, Mallat ME, Hussein R, et al. Posterior lingual lidocaine: a novel method to improve tolerance in upper gastrointestinal endoscopy. World J Gastroenterol. 2011; 17:5191–5196.

15. Ayoub C, Skoury A, Abdul-Baki H, Nasr V, Soweid A. Lidocaine lollipop as single-agent anesthesia in upper GI endoscopy. Gastrointest Endosc. 2007; 66:786–793.

16. Salale N, Treldal C, Mogensen S, Rasmussen M, Petersen J, Andersen O, et al. Bupivacaine lozenge compared with lidocaine spray as topical pharyngeal anesthetic before unsedated upper gastrointestinal endoscopy: a randomized, controlled trial. Clin Med Insights Gastroenterol. 2014; 7:55–59.

17. Chan CK, Fok KL, Poon CM. Flavored anesthetic lozenge versus Xylocaine spray used as topical pharyngeal anesthesia for unsedated esophagogastroduodenoscopy: a randomized placebo-controlled trial. Surg Endosc. 2010; 24:897–901.

18. İbiş M, Arhan M, İbiş T, Önal İK, Erdal H, Utku ÖG. Lidocaine versus lidocaine plus benzydamine as a topical anesthesia regimen for unsedated upper gastrointestinal endoscopy: a comparison study. Turk J Gastroenterol. 2015; 26:224–227.

19. Motamed F, Aminpour Y, Hashemian H, Soltani AE, Najafi M, Farahmand F. Midazolam-ketamine combination for moderate sedation in upper GI endoscopy. J Pediatr Gastroenterol Nutr. 2012; 54:422–426.

20. Brecelj J, Trop TK, Orel R. Ketamine with and without midazolam for gastrointestinal endoscopies in children. J Pediatr Gastroenterol Nutr. 2012; 54:748–752.

21. Langston WT, Wathen JE, Roback MG, Bajaj L. Effect of ondansetron on the incidence of vomiting associated with ketamine sedation in children: a double-blind, randomized, placebo-controlled trial. Ann Emerg Med. 2008; 52:30–34.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download