Abstract
Purpose
Congenital esophageal atresia (CES) is a rare congenital disease. The severity of symptoms is variable; thus, diagnosis is difficult and tends to be delayed. CES is frequently accompanied by esophageal atresia (EA) with/without tracheoesophageal fistula (TEF). We investigated the characteristics of CES by reviewing our experience with CES patients and researched the differences between CES with EA-TEF and isolated CES.
Methods
A total of 31 patients underwent operations for CES were reviewed retrospectively. The patients were divided into two groups according to the association with EA-TEF, and compared the differences.
Results
Sixteen boys and 15 girls were included. The mean age at symptom onset was 8 months old, and the mean age at diagnosis was 21 months old. Nine patients with EA-TEF were included group A, whereas the other 22 patients were assigned to group B. There were no differences in sex, gestational age, associated anomalies and pathologic results between the groups. In group A, the age at diagnosis and age at surgery were younger than in group B despite the age at symptom occurrence being similar. Postoperative complications occurred only in group A.
Conclusion
In this study, symptoms occurred during the weaning period, and vomiting was the most frequent symptom. CES patients with EA-TEF tended to be diagnosed and treated earlier despite the age at symptom occurrence being similar. CES patients with EA-TEF had more postoperative complications; therefore, greater attention should be paid during the postoperative period.
Congenital esophageal stenosis (CES) is a congenital stenosis of the esophagus due to intrinsic abnormalities of the esophageal wall. CES is a rare congenital anomaly, and its incidence has been estimated at 1 per 25,000 to 50,000 live births [1234]. Currently, the most widely used classification of CES, according to histologic abnormality of the esophageal wall, consists of three categories including tracheobronchial remnants (TBR), fibromuscular hypertrophy (FMH) and membranous diaphragm (MD) [5]. The symptoms depend on the location and the degree of the stenosis, and the variety of symptoms sometimes causes delays in diagnosis [6]. The treatment strategy depends on the etiology. TBR patients are recommended for resection, whereas FMH and MD patients are primary recommended for balloon dilatation [7].
CES and esophageal atresia (EA) with/without tracheoesophageal fistula (TEF) are assumed to have a similar etiology, and CES is frequently accompanied by EA-TEF. The reported coincidence rate was approximately 25% [4]. During the embryonic period, abnormal development between the respiratory and digestive tubes causes a TEF, and abnormal incorporation of the respiratory tissue into the esophageal wall results in CES [18]. When CES is associated with EA-TEF, they can sometimes show different clinical courses than isolated CES, i.e., CES without EA-TEF. Although CES has been studied in infants and young children for many years, there have been few studies of CES associated with EA-TEF or of the differences between isolated CES and CES with EA-TEF.
The purposes of this study were to review our experience in CES patients over 23 years at a single center and to research the differences between CES with EA-TEF and isolated CES.
From 1990 to 2012, 31 patients underwent surgery for CES at Seoul National University Children's Hospital. We performed retrospective medical record reviews regarding the patients' demographic information such as sex, gestational age, birth weight, weight at operation, age at diagnosis and operation, associated anomalies, and clinical symptoms. The diagnostic methods, management and results were also reviewed. The location and degree of CES were evaluated on esophagography. The location was described as the proximal, middle or distal third of the esophagus. The degree of stenosis on esophagography was assessed as mild (when the diameter of the esophagus at the CES level was greater than two-thirds of the normally distensible esophagus diameter above or below it), moderate (when the diameter was less than two-thirds but greater than one-third of the normal esophagus diameter), or severe (when the diameter was less than one-third of the normal esophagus diameter).
We divided the patients into two groups based on CES association with EA-TEF. CES patients with EA-TEF were classified as group A, whereas isolated CES patients were classified as group B. Patient demographics and therapeutic results were compared between the groups. The chi-square test, Kruskal-Wallis test and Mann-Whitney U-test were used with SPSS software (ver. 14.0; SPSS Inc., Chicago, IL, USA) for statistical analysis. A p-value less than 0.05 was considered statistically significant.
The patient demographics and the results of the diagnostic examinations of the 31 patients are summarized in Table 1. There were 16 boys and 15 girls included in the study. Twenty-nine patients were born at more than 36 weeks of gestational age, and 2 patients were born before 36 weeks: at 29 weeks 6 days and 34 weeks, respectively. Twenty-six patients weighed more than 2,500 g at birth, and 5 patients weighed less than 2,500 g. The age at first symptom presentation ranged from 1 day to 10 years old (median, 8 months), and the age at diagnosis ranged from 8 days to 12 years old (median, 21 months). The median interval from first symptom occurrence to diagnosis was 54 days (range from 1 day to 1 year). The median weight of the patients at surgery was 11.7 kg, ranging from 5.7 kg to 45.9 kg. Seventeen patients had associated anomalies, and some patients had multiple anomalies. Twenty-nine patients presented vomiting, the most common presenting symptom. Foreign bodies or food material impaction (7 patients), frequent respiratory tract infections (5 patients) and dysphagia (5 patients) were also commonly presenting symptoms. The median follow-up period was 2.32 years (range, 3 months to 16 years).
Preoperative diagnosis was based on esophagography and endoscopy. Esophagography was performed in all 31 cases for initial study and endoscopy in 22 cases for checking the stenosis and distinguishing CES from peptic stenosis. The site of stricture was found in the lower 3rd portion of the esophagus in all of the patients (Fig. 1) except for one patient with double CES. This patient had CES in the middle portion and lower 3rd portion of the esophagus (Fig. 2). The degree of stenosis was severe in 17 cases and moderate in 15 cases. There were no mild cases. The age at first symptom presentation and the degree of stenosis showed no statistical significance (p=0.834).
All 31 patients underwent resection of the stenotic segment and end-to-end anastomosis. In 30 cases, we performed an abdominal approach, and in 1 case, a thoracic approach was undertaken. Four children were initially treated with esophageal balloon dilatation for CES, which was ineffective in all 4 patients. In the double CES patient, only the distal CES, which was classified as severe, was excised using an abdominal approach.
There were some complications in 3 patients. Anastomosis site leakage occurred in 1 patient and was managed non-operatively. Anastomosis site stricture occurred in 1 patient, and this patient was treated with esophageal balloon dilatation. One patient had symptomatic gastroesophageal reflux disease postoperatively, and he was managed with conservative therapy.
Twenty-seven of 31 patients were feeding well after surgery. Four patients had feeding intolerance after surgery with no relationship to the surgical complications. Among these four patients, 3 were suspected of having an antral web on postoperative contrast studies, and one patient was presumptively diagnosed with achalasia by esophageal manometry. Of the 3 patients suspected of having antral webs, 2 patients gradually improved in their feeding intolerance without treatment, but the other patient underwent antral web plasty. The one achalasia patient was managed with esophageal balloon dilatation, and his symptoms improved (Fig. 3).
Histologic examination revealed TBR in 29 patients and FMH in 2 patients. There were no patients with MD in this study.
Nine patients with EA-TEF were included in group A, whereas the other 22 isolated CES patients were assigned to group B. All of the group A patients had EA with distal TEF and underwent end-to-end anastomosis of the esophagus and closure of the TEF soon after birth. We regularly examined the all EA-TEF patients after surgery with esophagography for the presence of leakage or stricture of the anastomosis site.
There were no significant differences in general epidemiologic information. The sex ratio and gestational age were similar in both groups. The birth weight of group A was smaller than that of group B (p=0.04). The presences of associated anomalies (p=0.375) and pathologic findings (p=0.503) also did not show statistically significant differences between groups A and B.
In both groups, the age at first symptom presentation was similar, but the age at diagnosis was much younger in group A (p=0.001). The age at surgery was younger in group A (p=0.01), and the weight at surgery was also less in group A (p=0.029).
Postoperative outcomes seemed to be better in group B than in group A. Postoperative complications only occurred in group A. Feeding intolerance, showing no relationships with surgical complications, occurred in 2 patients each in both groups, but the progression was different. The group B patients experienced spontaneous resolution of symptoms with observation only, whereas the group A patients were diagnosed with other diseases and underwent additional treatment. The aforementioned achalasia patient and the patient who underwent web plasty due to an antral web were included in group A (Table 2).
According to previous studies, CES has shown a slightly male-dominant incidence [9]. From a review of 132 patients from 12 articles recording sex, boys also showed a slight predominance with a ratio of 70:62 boys to girls [125789101112131415]. Most of patients feed well at birth and gradually show feeding intolerance, even as adults [16]. Typically, the onset of symptoms is known to correspond to the weaning period and the introduction of solid food [12]. As a result, information about birth, including gestational age, weight and mode of delivery, is frequently archived. In our study, the male-to-female ratio showed slight male predominance, and the mean age at symptom occurrence was 8 months old, similar to the results of previous studies. Most of patients were born at full term and weighed more than 2,500 g.
Esophagography is the most widely used tool for the diagnosis of CES, and it shows the location, shape and degree of stenosis. CES has typical findings of luminal narrowing with proximal dilatation of the esophagus on esophagography [2111217]. The pathologic type is important for planning the treatment strategy, but with esophagography, differential diagnosis among TBR, FMH and MD is impossible [3]. Endoscopy is also a commonly applied diagnostic modality, showing the location and shape of CES. Acquired esophageal stenosis, caused by reflux esophagitis or achalasia, is sometimes mimicked by CES on esophagography and endoscopy [11]. However, on endoscopy, pathologic type also cannot be distinguished. Endoscopic ultrasonography (EUS) has been adopted, and differential diagnosis could be performed with it [7]. Esophageal manometry and 24-hour esophageal pH monitoring were also applied for the differentiation of CES from other types of acquired esophageal stenosis [11]. We also used esophagography as the method of screening in the most of the patients. Three patients, whose first symptom was food or foreign body impaction, underwent endoscopic examinations first for foreign body removal. Unfortunately, in our center, EUS for infants and small children was impossible due to relatively large size of the endoscope until 2012. However, on EUS, the pathologic type can currently be obtained, and the therapeutic plan can also be decided in many cases [4].
The treatment strategy for CES depends on the pathologic type. For MD patients, balloon dilatation and endoscopic incision can result in improvement [37]. In FMH patients, esophageal dilatation has shown improvements, but it does not always have an effect. In some studies, TBR patients experience no benefit from dilatation, and resection of the stenotic segment and anastomosis suggest the treatments of choice [1115]. Other study showed complete resolution of stenosis and symptoms after dilatation alone, even for TBR patients [18]. Yet another study suggested that, for TBR patients, balloon dilatation might be effective, but esophageal perforations after balloon dilatation only occurred in TBR patients [19]. In our study, 4 patients underwent balloon dilatation, but none of them experienced improvement. They underwent resection and were eventually diagnosed with TBR. Because only patients who underwent surgery were included in this study, most of the patients had TBR and more severe than moderate degree, balloon dilatation might seem to be an ineffective therapy for CES.
We operated on 30 of 31 patients using an abdominal approach because the lesions were located in the distal third of the esophagus, except for one patient with double CES. In the one patient who underwent a thoracic approach, although the lesion was located in the distal esophagus, the distance from gastroesophageal junction to the lesion was relatively long. The TBR type of CES, constituting most of our cases, is known to be located generally within 3 cm of the gastroesophageal junction [17]. We believe that the approach should be chosen according to the distance from the lesion to the gastroesophageal junction.
Postoperative feeding intolerance without obvious complications can occur due to various causes, such as the presences of co-existing disease, postoperative complications and the basic characteristics of CES patients. CES patients commonly have impaired esophageal motility and gastroesophageal reflux [20]. Symptoms such as vomiting and regurgitation could persist after successful surgery for CES, and anti-reflux operations, including Nissen fundoplication, might sometimes be necessary to overcome feeding intolerance [911]. Two patients who were suspected of having antral webs and recovered spontaneously might have had complications from an abdominal approach. With the abdominal approach, injury of the vagus nerve could occur, so pyloroplasty was occasionally needed [15]. In the operating room, we preserved the vagus nerve, but microscopic injuries could not be excluded. In our study, one patient simultaneously had three esophageal diseases, EA-TEF, CES and achalasia. He was the first reported patient to have three diseases [21]. This patient required consideration for the presence of other esophageal diseases when feeding intolerance occurred postoperatively.
We routinely examined the EA-TEF patients with esophagography for the presence of leakage or stricture of the anastomosis site, and for follow-up, periodical esophagography was performed. According to a previous study of our center, 22 CES patients, including mild cases, were found among 187 EA-TEF patients [21]. We observed these patients closely, and when symptoms occurred, they received treatment promptly. In contrast, the isolated CES patients were firstly examined after experiencing symptoms that generally began after 6 months old, during the weaning period. This difference in timing was the reason that the group A patients were diagnosed and operated on at younger ages.
However, in the CES patients with EA-TEF, if the diagnosis of CES was missed at an early age, the patients also experienced delayed diagnosis. In these patients, the diagnosis rate of CES on initial esophagography was reported as only 62% [19]. Diagnosis of CES on esophagography is sometimes very difficult. When CES is located in only the distal part of the anastomosis site of EA at a short distance, CES is rarely found. In particular, with the presence of stricture at the anastomosis site of EA, leading to proximal severe esophageal dilatation, the narrowing due to CES could be confused with a normal esophagus, and the diagnosis could be delayed [21]. Our diagnosis rate of CES on initial esophagography was 55.6% (5/9) (Table 3). After careful retrospective review of them, 2 cases of CES could be diagnosed at follow-up test before the age of diagnosis. We think our low diagnosis rate result from that we only focused on stricture and patency of anastomosis site at initial esophagography.
Postoperative complications only occurred in the patients with EA-TEF. These patients might have had short lengths of the esophagus and intra-thoracic adhesions. We mostly operated on these patients with an abdominal approach, but in another study in which the thoracic approach was performed in a relatively high proportion of patients, it was reported that, in patients with previous histories of thoracotomy such as surgery for EA-TEF, severe adhesions and changes in pleural injury might present [2]. We estimated that these conditions would lead to ischemia at the anastomosis site, causing tension or making the operation more difficult. Group A patients performed operation for CES earlier than group B patients. Younger patients are more likely to develop complications compare to older patients. These factors could also cause complications, including anastomosis leakage and stricture.
Because most of patients in this study had TBR, only a few FMH patients and no MD patients were included. These proportions were much smaller than in other studies. Thus, there were some limitations for the evaluation of the overall characteristics of CES. In the future, a larger sample size is required, including patients treated with endoscopic procedures or with dilatation alone.
In our study, symptoms occurred during the weaning period, and vomiting was the most frequent symptom. Most of the patients had pathologic diagnoses of TBR.
CES patients with EA-TEF tended to be diagnosed and treated earlier due to postoperative esophagography for EA-TEF, despite the age at symptom occurrence being similar. CES patients with EA-TEF had more postoperative complications, so greater care should be undertaken when operating on CES patients with EA-TEF.
Figures and Tables
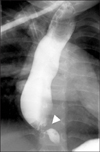 | Fig. 1Esophagography of a patient with congenital esophageal stenosis. The site of stricture was found in the lower 3rd portion of the esophagus (arrowhead). |
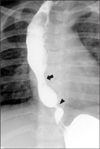 | Fig. 2Esophagography of a patient with double congenital esophageal stenosis. The sites of stricture were founded in the middle portion (arrow) and the lower 3rd portion of the esophagus (arrowhead). |
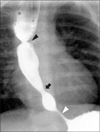 | Fig. 3Esophagography of a patient who had esophageal atresia, congenital esophageal stenosis and achalasia simultaneously (black arrowhead: anastomosis site of esophageal atresia, black arrow: congenital esophageal stenosis, white arrowhead: site of achalasia). |
References
1. Neilson IR, Croitoru DP, Guttman FM, Youssef S, Laberge JM. Distal congenital esophageal stenosis associated with esophageal atresia. J Pediatr Surg. 1991; 26:478–481. discussion 481-2.

2. Nam SH, Kim DY, Kim SC, Kim IK. The diagnosis and treatment of congenital esophageal stenosis. J Korean Surg Soc. 2009; 76:383–387.

3. Lee KS. Preoperative diagnosis of congenital esophageal stenosis caused by tracheobronchial remnants using miniprobe endoscopic ultrasonography in a child. Pediatr Gastroenterol Hepatol Nutr. 2012; 15:52–56.

4. Terui K, Saito T, Mitsunaga T, Nakata M, Yoshida H. Endoscopic management for congenital esophageal stenosis: a systematic review. World J Gastrointest Endosc. 2015; 7:183–191.

5. Nihoul-Fékété C, De Backer A, Lortat-Jacob S, Pellerin D. Congenital esophageal stenosis. Pediatr Surg Int. 1987; 2:86–92.

6. Ramesh JC, Ramanujam TM, Jayaram G. Congenital esophageal stenosis: report of three cases, literature review, and a proposed classification. Pediatr Surg Int. 2001; 17:188–192.

7. Takamizawa S, Tsugawa C, Mouri N, Satoh S, Kanegawa K, Nishijima E, et al. Congenital esophageal stenosis: therapeutic strategy based on etiology. J Pediatr Surg. 2002; 37:197–201.

8. Kawahara H, Imura K, Yagi M, Kubota A. Clinical characteristics of congenital esophageal stenosis distal to associated esophageal atresia. Surgery. 2001; 129:29–38.

9. Zhao LL, Hsieh WS, Hsu WM. Congenital esophageal stenosis owing to ectopic tracheobronchial remnants. J Pediatr Surg. 2004; 39:1183–1187.

10. Elhalaby EA, Elbarbary MM, Hashish AA, Kaddah SN, Hamza AF, Waheeb SM, et al. Congenital esophageal stenosis: to dilate or to resect. Ann Pediatr Surg. 2006; 2:2–9.
11. Amae S, Nio M, Kamiyama T, Ishii T, Yoshida S, Hayashi Y, et al. Clinical characteristics and management of congenital esophageal stenosis: a report on 14 cases. J Pediatr Surg. 2003; 38:565–570.

12. Murphy SG, Yazbeck S, Russo P. Isolated congenital esophageal stenosis. J Pediatr Surg. 1995; 30:1238–1241.

13. Nishina T, Tsuchida Y, Saito S. Congenital esophageal stenosis due to tracheobronchial remnants and its associated anomalies. J Pediatr Surg. 1981; 16:190–193.

14. Singaram C, Sweet MA, Gaumnitz EA, Cameron AJ, Camilleri M. Peptidergic and nitrinergic denervation in congenital esophageal stenosis. Gastroenterology. 1995; 109:275–281.

15. Vasudevan SA, Kerendi F, Lee H, Ricketts RR. Management of congenital esophageal stenosis. J Pediatr Surg. 2002; 37:1024–1026.

16. Oh CH, Levine MS, Katzka DA, Rubesin SE, Pinheiro LW, Amygdalos MA, et al. Congenital esophageal stenosis in adults: clinical and radiographic findings in seven patients. AJR Am J Roentgenol. 2001; 176:1179–1182.
17. Nemolato S, De Hertogh G, Van Eyken P, Faa G, Geboes K. Oesophageal tracheobronchial remnants. Gastroenterol Clin Biol. 2008; 32:779–781.

18. Romeo E, Foschia F, de Angelis P, Caldaro T, Federici di, Gambitta R, et al. Endoscopic management of congenital esophageal stenosis. J Pediatr Surg. 2011; 46:838–841.

19. Michaud L, Coutenier F, Podevin G, Bonnard A, Becmeur F, Khen-Dunlop N, et al. Characteristics and management of congenital esophageal stenosis: findings from a multicenter study. Orphanet J Rare Dis. 2013; 8:186.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download