This article has been
cited by other articles in ScienceCentral.
Abstract
Intestinal hypoganglionosis is a rare innervation disorder that provides numerous nutritional, medical and surgical challenges. In this case report, we present a case of a newborn with intestinal hypoganglionosis leading to intestinal failure and intestinal failure-associated liver disease who responded to Omegaven™, a fat emulsion comprised of omega-3 fatty acids. Omegaven™ has been shown to be beneficial in the management of cholestatic liver injury. Clinical success with Omegaven™ was seen in this patient with a clear decrease in aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and complete resolution of cholestasis with a direct bilirubin of zero within two weeks of initiation of Omegaven™. No current guidelines for the diagnosis and management of hypoganglionosis are available. We recommend a multidisciplinary approach and the use of novel therapies such as fat emulsions composed of omega-3 fatty acids for improved patient outcomes. Appropriate compassionate use protocols should be obtained from the Food and Drug Administration prior to initiation of Omegaven™.
Go to :

Keywords: Hirschsprung disease, Omega-3 fatty acids, Liver diseases, Total parenteral nutrition
INTRODUCTION
Intestinal hypoganglionosis is an innervation disorder that provides diagnostic and management challenges secondary to ill-defined diagnostic criteria [
1]. A total of 92 cases of isolated hypoganglionosis have been reported with a male to female prevalence of 3:1, and a median age at diagnosis of 4.85 years. Of these, 29 patients (31.5%) had neonatal onset of symptoms [
2].
Clinical presentation is variable as well as age at diagnosis. There is no data on symptom incidence in the neonatal period including failure to pass meconium as seen in Hirschsprung disease (HD). Management is surgical and entails the resection of the affected segment [
2].
Outcomes for patients with hypoganglionosis are controversial and highly variable. Mortality rate for isolated hypoganglionosis is reported at 8% across all age groups [
2]. Outcomes are highly dependent on the length of the affected bowel, prolonged use of total parenteral nutrition (TPN), and associated infections [
1].
We present a case of a newborn with intestinal hypoganglionosis leading to intestinal failure and progressive intestinal failure-associated liver disease (IFALD), who responded to Omegaven™ (Fresenius Kabi, Bad Homburg vdh, Germany), a fat emulsion comprised of omega-3 fatty acids. Omegaven™ has been shown to be beneficial in the management of cholestatic liver injury [
34]. These emulsions inhibit de novo lipogenesis, reduce arachidonic acid-derived inflammatory mediators, prevent essential fatty acid deficiency, and improve clearance of lipids from the serum [
35].
Go to :

CASE REPORT
A Caucasian male newborn infant born at term presenting to the neonatal intensive care unit with respiratory distress developed multiple episodes of bilious emesis and failed to pass meconium within the first 72 hours of life. Physical examination on admission was only notable for decreased air entry bilaterally on lung auscultation. Abdominal examination revealed a soft, non-distended abdomen with no organomegaly. Initial laboratory workup including a complete metabolic panel and a complete blood count were within normal limits for age. Abdominal radiography showed dilated loops of bowel and water soluble enema was significant for microcolon (
Fig. 1).
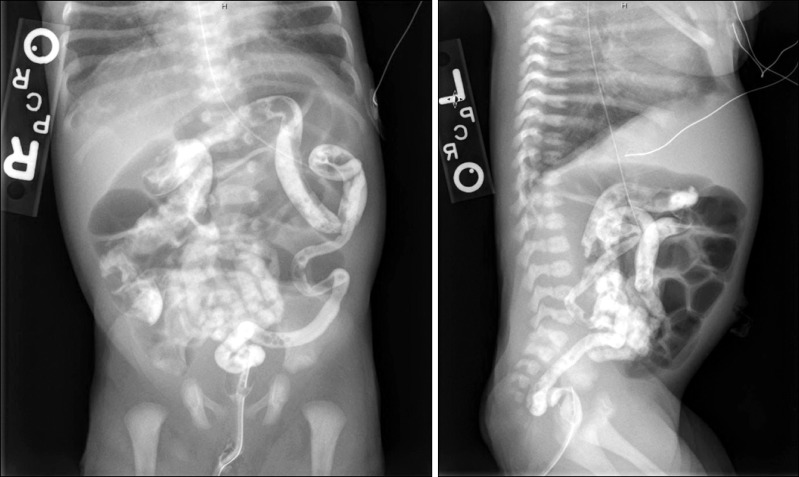 | Fig. 1Contrast enema obtained on day of life four. The colon is diffusely small in caliber. Microcolon is more prominent in the transverse colon to rectum, and somewhat less so in the cecum right colon.
|
An exploratory laparotomy was undertaken and there was an apparent caliber differential in the ileum approximately 40 cm proximal to the ileocecal valve. However, this did not correlate to a histologic HD transition zone, as ileum biopsies and appendectomy showed present ganglion cells, possibly decreased in density. A subsequent rectal suction biopsy showed submucosal ganglion cells and adequate Calretinin immunoreactive pattern, confidently excluding HD. During a second laparotomy, multiple full and partial thickness biopsies revealed decreased number and small and immature ganglion cells in the myenteric plexus throughout the colon and distal ileum (
Fig. 2). Ganglion cells in the submucosal plexus appeared larger and more mature. No hypertrophic nerves were identified, but the number of ganglion cells and ganglia groups appeared decreased. This hypoganglionosis involved the entire small intestine, appendix and colon sampled for microscopic evaluation.
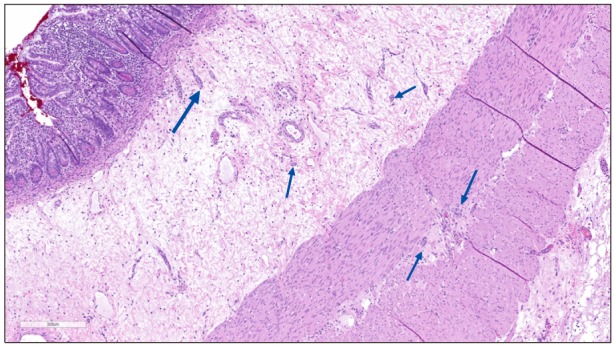 | Fig. 2Full thickness section of ileum. Limited numbers and small clusters of ganglion cells (arrows) seen in submucosal and myenteric plexuses. No fibrosis, nerve proliferation, neuronal dysplasia or myopathic changes identified. Note absence of ganglia in adjacent myenteric zone (H&E, ×100).
|
Initial surgical management included ileostomy and mucus fistula creation at the site of the intestinal caliber change. Post-operatively, the patient was unable to tolerate enteral feeds due to bilious emesis, high gastric output, low ileostomy output, intestinal dilatation and bacterial overgrowth. During two subsequent laparotomies the patient had gastrostomy tube placement, a jejunostomy tube placement, lysis of adhesions, and relocation of the ileostomy 20 cm more proximally in hopes of allowing more intestinal throughput. Adaptation to tolerate enteral feeds was slow and attempts at refeeding proximal ostomy output into the distal mucus fistula were unsuccessful. Care was managed by a multidisciplinary team.
Thus, the patient required nutritional support parenterally. Prolonged parenteral nutrition along with other risk factors predisposed the patient to and led to IFALD, with a peak conjugated bilirubin of 4.7 mg/dL.
Hospital course was complicated by central line infections and a urinary tract infection. An ethanol lock protocol for central line was initiated to help reduce the incidence of central line infections.
The combination of prolonged TPN use, episodes of sepsis and functional short bowel led to progressive IFALD. Transaminitis and hyperbilirubinemia were observed within weeks after starting TPN (
Fig. 3). Peak aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 253 and 261 IU/L respectively, peak alkaline phosphatase was 361 IU/L. Given repeated failure to initiate and maintain enteral feeds, alternative interventions were indicated. Trace elements excreted in the bile including copper and manganese were cycled. Intravenous lipids were weaned to 1 g/kg/d and discontinued when Food and Drug Administration (FDA) approval was granted for compassionate administration of Omegaven™.
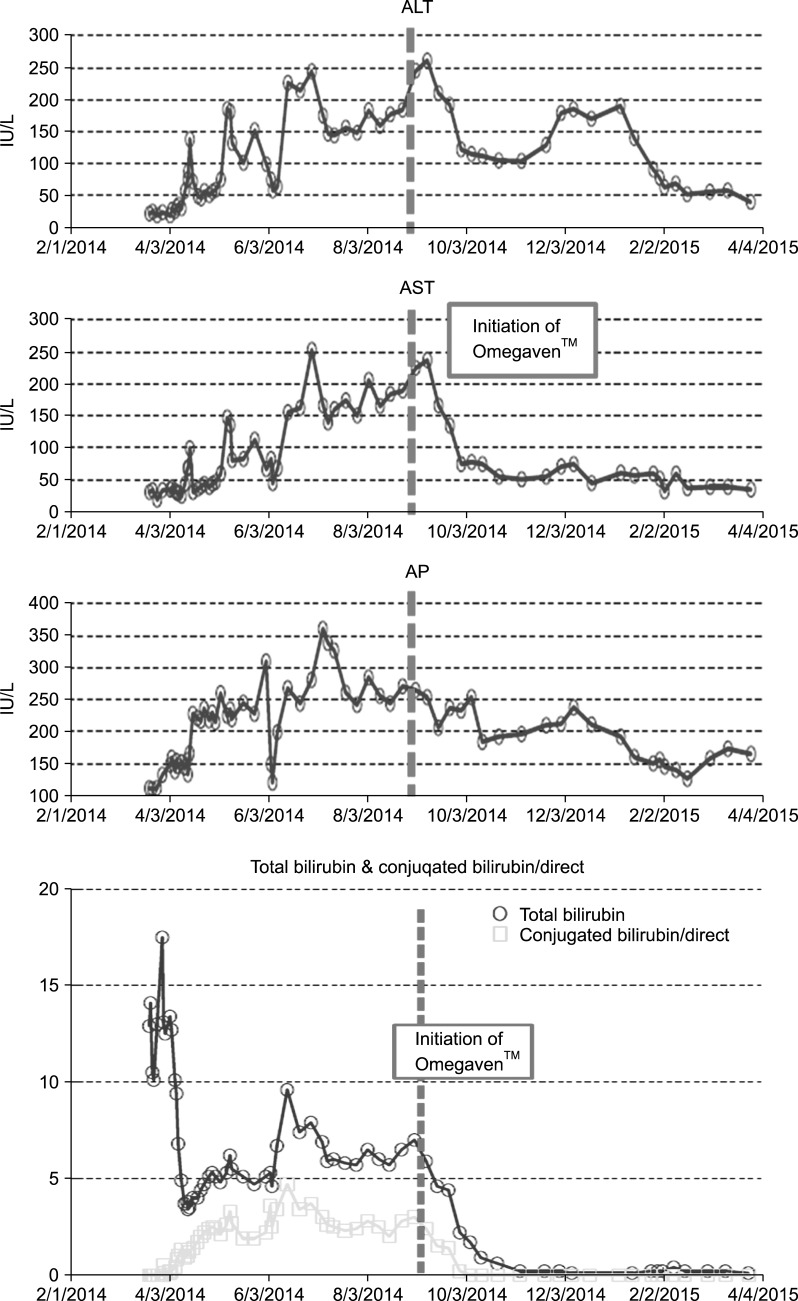 | Fig. 3Clinical measures indicative of intestinal failure associated liver disease. Pertinent laboratory values prior to and post administration of Omegaven™ and concurrent discontinuation of intravenous lipids. ALT: alanine aminotransferase, AST: aspartate aminotransferase, AP: alkaline phosphatase.
|
Clinical success with Omegaven™ was seen in this patient with a clear decrease in AST, ALT and complete resolution of cholestasis with a direct bilirubin of zero within two weeks of initiation of Omegaven™ (
Fig. 3). This fat emulsion was administered for a total of 40 days. No adverse effects were observed and the patient was discharged on enteral feeds and parenteral nutrition, which was also discontinued shortly after discharge.
At 17 months follow up, patient remains off parenteral nutrition. He is currently receiving 60% of his diet orally. Supplements are given via gastric tube. The central line was surgically removed once the patient was meeting caloric goals enterally. He has not required any further surgical interventions since discharge. He is at the 40 percentile for weight and 39 percentile for height on the Centers for Disease Control and World Health Organization combined growth chart.
Go to :

DISCUSSION
Care of a patient with hypoganglionosis is complex and requires a multidisciplinary approach. Numerous elements of care led to a favorable outcome in our patient including decision making guided by a multidisciplinary intestinal rehabilitation team. The team focused on meticulous management of parenteral nutrition, use of standardized enteral management guidelines for feeding initiation, advancement and tolerance of feedings, treatment of short bowel bacterial overgrowth, initiation of ethanol locks for preventing central line infections, and use of Omegaven™ to prevent hepatotoxicity.
It is estimated that 40-60% of children who receive prolonged TPN are at risk for developing IFALD. The mechanism of IFALD remains unknown but is deemed to be multifactorial with contributors including components of parenteral nutrition, sepsis and lack of enterally simulated bile flow [
6]. Infants and neonates are at higher risk for developing severe and progressive IFALD [
7].
Standard of care intralipid emulsions are rich in omega-6 fatty acids which have pro-inflammatory effects and impair triglyceride export. These have thus been implicated in the pathogenesis of IFALD [
68]. On the other hand, omega-3 emulsions such as Omegaven™ have anti-inflammatory effects and appear to offer hepatic protection [
56]. Our patient received omega-6 fatty acid rich emulsions prior to developing IFALD. As he failed progression of enteral feeds and standard of care treatment with reduction and cycling of intralipid administration, compassionate use of an omega-3 fatty acid suspension was approved by the FDA. IFALD resolved within two weeks of starting an emulsion rich in omega-3 fatty acids and discontinuation of omega-6 fatty acid rich emulsions.
Comparable clinical success has been reported in a case by Calhoun and Sullivan [
9]. who describe resolution of parenteral nutrition induced cholestasis in a 17 months old infant with short gut upon initiation of compassionate use of Omegaven™. Similar evidence has been reported in human, animal, and cell-based studies [
469].
Short bowel syndrome is another risk factor for IFALD [
10]. Although our patient had normal intestinal length, his very proximal enterostomy and the inability to use the distal intestine by a refeeding protocol effectively made him a short bowel syndrome patient. Thus, infants diagnosed with intestinal hypoganglionosis are inherently at an increased risk for developing cholestasis.
Episodes of sepsis such as urinary tract infections and catheter related infections also increase the risk for IFALD [
11]. Bacterial overgrowth in the small intestine may also lead to bacterial translocation which has also been shown to play a role in the pathophysiology of IFALD. Our patient suffered from both episodes of sepsis as well as from bacterial overgrowth in the small intestine.
While there are no current guidelines for the diagnosis and management of hypoganglionosis, we recommend a multidisciplinary approach and the use of novel therapies such as fat emulsions composed of omega-3 fatty acids.
Go to :

ACKNOWLEDGEMENTS
The authors would like to kindly thank pathologists Dr. Hector Monforte and Dr. Ignacio Gonzalez-Gomez for their contributions to this case report.
Go to :

References
1. Schäppi MG, Staiano A, Milla PJ, Smith VV, Dias JA, Heuschkel R, et al. A practical guide for the diagnosis of primary enteric nervous system disorders. J Pediatr Gastroenterol Nutr. 2013; 57:677–686. PMID:
24177787.

2. Dingemann J, Puri P. Isolated hypoganglionosis: systematic review of a rare intestinal innervation defect. Pediatr Surg Int. 2010; 26:1111–1115. PMID:
20721562.

3. Le HD, de Meijer VE, Robinson EM, Zurakowski D, Potemkin AK, Arsenault DA, et al. Parenteral fish-oil-based lipid emulsion improves fatty acid profiles and lipids in parenteral nutrition-dependent children. Am J Clin Nutr. 2011; 94:749–758. PMID:
21775562.

4. Park HW, Lee NM, Kim JH, Kim KS, Kim SN. Parenteral fish oil-containing lipid emulsions may reverse parenteral nutrition-associated cholestasis in neonates: a systematic review and meta-analysis. J Nutr. 2015; 145:277–283. PMID:
25644348.

5. Raptis DA, Limani P, Jang JH, Ungethüm U, Tschuor C, Graf R, et al. GPR120 on Kupffer cells mediates hepatoprotective effects of ω3-fatty acids. J Hepatol. 2014; 60:625–632. PMID:
24262133.

6. Venick RS, Calkins K. The impact of intravenous fish oil emulsions on pediatric intestinal failure-associated liver disease. Curr Opin Organ Transplant. 2011; 16:306–311. PMID:
21505340.

7. Kelly DA. Liver complications of pediatric parenteral nutrition--epidemiology. Nutrition. 1998; 14:153–157. PMID:
9437702.

8. Lacaille F, Gupte G, Colomb V, D'Antiga L, Hartman C, Hojsak I, et al. Intestinal failure-associated liver disease: a position paper of the ESPGHAN working group of intestinal failure and intestinal transplantation. J Pediatr Gastroenterol Nutr. 2015; 60:272–283. PMID:
25272324.
9. Calhoun AW, Sullivan JE. Omegaven for the treatment of parenteral nutrition associated liver disease: a case study. J Ky Med Assoc. 2009; 107:55–57. PMID:
19263944.
10. Cooper A, Floyd TF, Ross AJ 3rd, Bishop HC, Templeton JM Jr, Ziegler MM. Morbidity and mortality of short-bowel syndrome acquired in infancy: an update. J Pediatr Surg. 1984; 19:711–718. PMID:
6440965.

11. Sondheimer JM, Asturias E, Cadnapaphornchai M. Infection and cholestasis in neonates with intestinal resection and long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. 1998; 27:131–137. PMID:
9702641.

Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download