Abstract
Purpose
Clinical symptoms associated with Clostridium difficile infection (CDI) can vary widely. Carrier state without apparent symptoms is relatively common during infancy. The objective of this study was to determine the association of C. difficile colonization with bowel habit change and the effect of C. difficile colonization treatment on restoration of normal bowel habit.
Methods
Between 2006 and 2014, infants at 1 to 12 months of age with diarrhea for more than 2 weeks who did not improve with conservative care were recruited from Gachon University Gil Medical Center. Infants who were followed up for at least 7 days were included. The presence or absence of C. difficile colonization, effect of metronidazole, and other medical records were reviewed. To determine the association between CDI and bowel habit change, logistic regression analysis was used.
Results
Of a total of 126 infants, 74 (58.7%) were male patients. Of the 126 patients, 27 (21.4%) had C. difficile colonization. Significant (p<0.05) risk factors for C. difficile colonization included artificial milk feeding (odds ratio [OR], 4.310; 95% confidence interval [CI], 1.564-11.878), prior rotavirus vaccination (OR, 4.322; 95% CI, 1.018-18.349), and antibiotic use (OR, 4.798; 95% CI, 1.430-16.101). There was improvement in bowel habit after metronidazole therapy (OR, 0.34; 95% CI, 0.15-0.79; p<0.05), regardless of the presence or absence of C. difficile colonization.
Chronic diarrhea in children shows age related spectrum. In infants and young children, chronic diarrhea is mainly related to persistent intestinal infections, intolerance to specific nutrients such as cow's milk protein, and functional diarrhea [1]. For infants, functional diarrhea is a frequent reason for consultation with ambulatory pediatrics and pediatric gastroenterology. Persistent diarrhea can lead to prolonged dehydration and malabsorption. Historically, health care-associated diarrhea among children has been attributed to viral pathogens [2]. However, recently, Clostridium difficile has been increasingly recognized as an important pathogen in children with persistent diarrhea [3].
C. difficile is a Gram-positive, obligate anaerobic, and spore-forming bacillus. It can produce toxins A and B which can cause intestinal diseases [4]. The prevalence of C. difficile colonization in adults ranges from 1% to 7%. However, it is higher in infants, ranging from 2% to 75% [56]. Although infants rarely develop C. difficile infection (CDI), C. difficile might act as an important reservoir of pathogen. Clinical symptoms of CDI are rare. Clinical manifestations of CDI are mild in infant, although high level of cytotoxin can cause severe colitis in adults [7].
Many factors can influence the chance of CDI. Recognized risk factors for the development of CDI include antimicrobial therapy, use of proton pump inhibitors, repeated enemas, use of diapers, prolonged nasogastric tube insertion, gastrostomy and jejunostomy tubes, underlying bowel disease, gastrointestinal tract surgery, renal insufficiency, and impaired humoralimmunity [8]. Previous antibiotic exposure has been recognized as the single most important risk factor for CDI development in children [9].
Diarrhea is a common symptom that may present during infancy. It is the most common cause of acute infectious diseases. Functional diarrhea is categorized as one of functional gastrointestinal disorders. It is defined by daily painless, recurrent passage of three or more large and unformed stools per day for four or more weeks, in otherwise healthy children [10]. There is no evidence of failure of children to thrive if adequate calories are consumed in the diet. The pathophysiology of functional diarrhea has not been clarified yet. However, the spectrum of the pathophysiology of functional diarrhea includes short feeding interval, immaturity of the gastrointestinal tract during infancy, frequent changes in formula such as weaning food and repeated infections of the intestinal tract [1].
The effect of digestive tract microorganisms on the host has not been confirmed. However diverse and densely populated gastrointestinal microbiota is essential for the regulation of host physiology and immune function. In addition, the complex gastrointestinal microbiota plays an important role in maintaining the intestinal immune system of the host [11]. CDI is an inflammatory condition in which homeostasis of the gut microbiota is disturbed, resulting in the presence of diarrhea in patients. Metronidazole can inhibit the synthesis of nucleic acid in microbial cells. It has been used as the standard therapy for managing moderate CDI in children as it is less expensive. It has the same efficacy as vancomycin [12]. The reason that metronidazole can be used to treat CDI might be due to the fact that metronidazole can recover the normal flora accompanied by the extinguishment of C. difficile.
The objective of this study was to determine the association of bowel habit change in infants with C. difficile colonization and the impact of management of C. difficile colonization with metronidazole on the restoration of normal bowel habit in infants.
All patients were Korean infants who visited pediatric gastrointestinal clinics of Gachon University Gil Medical Center from January 2006 to December 2014 due to increased stool frequency presenting as diarrhea or loose stool and diaper rash or dermatitis. Individuals aged from 1 to 12 months were subjected to C. difficile toxin A and toxin B test or C. difficile stool culture. Those patients who had an increase in stool frequency (more than 3 times/day) than prior bowel habit presenting as diarrhea or loose stool for more than 2 weeks despite conservative management for 2 weeks were included in the study. Exclusion criteria were as follows: lactose intolerance, a history of prior chronic diarrhea, other known congenital anomalies, prior gastrointestinal surgery, and those whose stools were not analyzed. The research protocol was approved by Gachon University Gil Medical Center Institutional Review Board (IRB no. GBIRB2016-163).
The diagnosis of C. difficile colonization was confirmed when C. difficile was identified in the stool culture or if rapid immunoassay was positive for C. difficile toxins A and B [513]. Bowel habit changes were defined when the frequency of stool was increased to at least 3 times a day compared to previous bowel habits or if the patient had loose or soft stools than usual which continued for more than 2 weeks. Restoration of bowel habit was defined as decreasing frequency of stool as before.
Clinical and environmental data were collected retrospectively for each patient based on information included in the medical chart obtained from the hospital database. Basic demographic data, including birth history (term of birth, birth weight), gender, age, body weight, type of feeding (exclusive breast feeding, exclusive formula feeding and a combination of breast and formula feeding), vaccination history, and medical treatment including the use of antibiotics were collected and analyzed for all patients. Former bowel habit checked at the time of the first visit was used as an indicator of change in bowel habit. If the infant failed to restore normal bowel habit within 7 days, the patient was judged as having bowel habit change.
In this study, patients who had visited several other primary clinics for sustained loose stool or diarrhea over two weeks with diaper rash and mild irritability were enrolled. We used metronidazole for these patients until stool test was confirmed as C. difficile negative. All patients were treated after obtained fully informed consent from their parents. Oral administration of metronidazole for 7 days was used as the initial treatment. After that, oral administration of metronidazole for 3-7 days was used if the results of stool culture or toxins were positive for C. difficile. The dose of metronidazole was 20 mg/kg per day.
We compared the clinical features between the two groups and analyzed the risk factors for C. difficile colonization during infancy. Naturally passed stool samples were collected from the diaper of each infant after defecation.
The extraction of toxin A and toxin B from C. difficile was conducted using enzyme linked fluorescent immunoassay (ELFA; VIDAS CDAB, Bio-Merieux SA, Lyon, France). For C. difficile stool culture, the collected stool was inoculated onto culture media of ChromID C. Difficile agar (Bio-Merieux SA) followed by anaerobic culture for 48 to 72 hours.
Descriptive values were expressed as the number of patients or mean±standard deviation. Categorical variables were expressed as proportions. Baseline characteristics of subjects were compared with chi-square test for categorical variables and Student t-test for continuous variables. A logistic regression analysis was used to adjust for risk factors associated with CDI and the effect of metronidazole. Statistical analyses were performed using PASW Statistics 18.0 for Windows (IBM Co., Armonk, NY, USA). A p-value of less than 0.05 was considered as statistically significant for all tests.
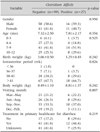
Of the 163 patients, 37 patients were excluded from the study: 7 patients had lactose intolerance, 2 patients had a history of prior gastrointestinal surgery, one patient had a history of chronic diarrhea, one patient had congenital malformation (pulmonary artery stenosis), 5 patients whose caretaker refused the stool examination, and 21 patients because of loss to follow up (Fig. 1). The remaining 126 patients (74; 58.7% males) who met the inclusion criteria were included for baseline evaluation. The mean age of patients was 7.43±2.43 months. These infants were divided into two groups according to the presence of C. difficile colonization. Ninety-nine (78.6%) infants were C. difficile colonization negative, whereas 27 (21.4%) infants were C. difficile colonization positive. Baseline characteristics between the presence and absence of C. difficile colonization subjects are summarized in Table 1. There was no significant difference in gender, age, weight at birth, gestational age, body weight, visit date, or treatment history in a primary health care clinic due to increased stool frequency.
In this study, C. difficile colonization was strongly associated with artificial milk formula (AMF) after excluding those patients who had formula feeding or a combination of breast and formula feeding. Infants who received AMF had a greater possibility of being positive for C. difficile colonization than infants who received breast milk (odds ratio [OR], 4.310; 95% confidence interval [CI], 1.564-11.878; p<0.05). Infants who received rotavirus vaccination and those with the use of antibiotics also had higher relative risk of C. difficile colonization (Table 2). Although the frequency of using probiotics was higher in C. difficile colonization negative group compared to that in the C. difficile colonization positive group, the difference was not statistically significant between the two. Regarding past history of receiving treatments in a private clinic, there were no significant difference between the two groups. Based on multivariate logistic regression analysis, the following factors had no statistically significant influence on the occurrence of C. difficile colonization: artificial milk feeding, vaccination against rotavirus, and the use of antibiotics.
When we analyzed the factors that influenced the change in bowel habit such as C. difficile colonization (p=0.863), AMF (p=0.251), rotavirus vaccination (p=0.707), use of antibiotics (p=0.842) and use of probiotics (p=0.786), the two groups showed no significant difference. However, administration of metronidazole (p=0.012) showed a statistically significant influence on change in bowel habit (Table 3).
Diarrhea is a very common symptom in infants. Most cases are acute disease associated with infectious agents. However, functional diarrhea can also be the cause of infant diarrhea. Therefore, clinicians must distinguish diseases from functional diarrhea. A careful history checking such as antibiotic use, exposure to enteric pathogens in daycare, growth problems, fever, rectal bleeding, and signs and symptoms of allergies should be performed.
Historically, CDI has been regarded as a nosocomial disease. However, it is now becoming increasingly clear that a significant percentage of CDI cases occur in the community, especially in younger patients without exposure to hospital or healthcare environment or with antimicrobial therapy [14]. In pediatric population, approximately 40% of patients with community-acquired CDI suffer from chronic gastrointestinal conditions [15]. Several studies have demonstrated that the use of antibiotics and exposure to healthcare facilities are associated with CDI [916]. In our study, we found that recent antibiotic exposure was a risk factor for CDI in children. If a change in normal colonic microbiota has occurred due to the use of antibiotics, C. difficile can easily colonize and produce toxins in the gastrointestinal tract of infants depending on the infant's general condition. Consequently, clinical manifestations such as diarrhea might appear.
In addition to history of antibiotic uses, this study showed that artificial milk feeding and history of rotavirus vaccination were also associated with CDI. Previous studies on fecal flora in babies have revealed that the count and incidence of C. difficile in artificial milk fed infants are significantly higher than those in breast-fed infants [17]. Several different mechanisms may contribute to the resistance of breast-fed infants to CDI. One reason for this could be that immunoglobulin fractions of breast milk can inhibit the binding of the toxin of C. difficile to its intestinal receptor [18].
We only found a trend of increased risk of C. difficile colonization during infancy following rotavirus vaccination. The formation of healthy intestinal flora is important for microbiota-induced host-homeostasis in the first months of life [19]. However, Ang et al. [20] have demonstrated that rotavirus vaccination has minimal or no effect on individual microbiota profiles of intestinal environment in three infants. Although changes of gut microbiome were not observed in infants, rotavirus vaccination could interact with intestinal mucosa and modify immunity during infancy. Hence, rotavirus vaccination might have an influence on CDI. No previous study has assessed the correlation between rotavirus vaccination and CDI. Therefore, further pediatric studies are needed to clarify the association between CDI and rotavirus vaccination.
Gastric acid suppressive drugs have been used to treat conditions such as dyspepsia, gastroesophageal reflux disease, stress ulcer prophylaxis, laryngopharyngeal reflux, duodenal ulceration, and Helicobacter pylori infection. In 2007, Leonard et al. [21] systematically reviewed 12 papers and evaluated the effect of previous use of acid suppressive drugs on CDI. They showed that the risk of CDI was greater with prior use of acid suppressive drugs. For this reason, it has been hypothesized that elevated gastric pH can influence the CDI risk by facilitating bacterial colonization of the upper gastrointestinal tract or the survival of vegetative phase of C. difficile in the stomach [9]. Several studies have found that gastric acid suppression is significantly associated with both initial CDI and CDI recurrence [2223]. On the other hand, the present study demonstrated that the presence of C. difficile colonization was not associated with use of gastric acid-suppressive agents (OR, 0.940; 95% CI, 0.278-2.940; p=0.866) (Table 2). Such discrepancy might be due to differences in sample size (which was small in this study compared to that in previous studies) and study targets.
Probiotics are live microorganisms. When administered in adequate dosage, probiotics can confer health benefit to the host. A wide variety of probiotics have been tested and used to prevent or treat CDI. Researchers have proposed that probiotics might be able to prevent diarrhea by maintaining the flora of the gut. However, the benefit of routine probiotic administration for preventing CDI is uncertain [24]. One meta-analysis has suggested that probiotics prophylaxis can result in a large reduction in CDI without increasing clinically important adverse events [25]. On the other hand, other researchers have suggested that routine use of probiotics might not be effective in preventing CDI [2627]. In the present study, colonization of C. difficile was not associated with the use of probiotics.
This study showed that the presence of C. difficile during infancy was not associated with infantile diarrhea. Asymptomatic intestinal colonization by C. difficile is common during early infancy. Our result is consistent with the finding of a previous study showing that there is no correlation between infantile diarrhea and colonization of C. difficile [28].
Infants with C. difficile have been regarded as a reservoir of C. difficile contamination. In the study of Loo et al. [29], asymptomatic C. difficile colonization was defined as positive stool culture for C. difficile in the absence of diarrhea. There has been no adequate evidence to support metronidazole therapy as routine management for fecal colonization by C. difficile [30]. On the other hand, this study demonstrated that there was improvement in bowel habit after metronidazole therapy regardless of C. difficile colonization.
Irritable bowel syndrome, a common functional gastrointestinal disorder, can be associated with gastrointestinal symptoms such as altered bowel function. Although the causes of irritable bowel syndrome remain undefined, several studies have increasingly suggested the roles of gut microbiota [3132]. A recent study by Lembo et al. [33] has demonstrated that rifaximin treatment is efficacious for diarrhea-predominant irritable bowel syndrome. They have suggested that rifaximin might have beneficial influence on the gut microbiota. Gut microbiota can prevent infection by harmful microorganisms through direct inhibition (releasing antimicrobial compounds), competition, or stimulating immune defenses of the host [34]. Consequently, because imbalances in gut microbiota have been associated with pathologies affecting human health, treatment with antibiotics such as metronidazole in infants with diarrhea is thought to alter infant's commensal intestinal flora that can inhibit the proliferation of C. difficile [13].
The limitations of our study include the use of retrospective data and a single center analysis. Another limitation of our study was that the sample size was small compared to that used in previous studies. Lastly, although enrolled and non-enrolled children did not appear to differ in terms of demographic characteristics, we could not rule out the possibility of unknown biases which might have affected our disease burden estimates. Therefore, further studies in other tertiary population-based cohorts are needed to validate our results.
In summary, there was no significant association between bowel habit change and C. difficile colonization during infancy. On the other hand, treatment of functional gastrointestinal disorder with metronidazole during infancy was correlated with improved gastrointestinal symptoms. These findings are useful for controlling symptoms in infants with chronic diarrhea due to the possibility of controlling gut microbiota.
Figures and Tables
References
1. Pezzella V, De Martino L, Passariello A, Cosenza L, Terrin G, Berni Canani R. Investigation of chronic diarrhoea in infancy. Early Hum Dev. 2013; 89:893–897.

2. Langley JM, LeBlanc JC, Hanakowski M, Goloubeva O. The role of Clostridium difficile and viruses as causes of nosocomial diarrhea in children. Infect Control Hosp Epidemiol. 2002; 23:660–664.

3. Kim J, Smathers SA, Prasad P, Leckerman KH, Coffin S, Zaoutis T. Epidemiological features of Clostridium difficile-associated disease among inpatients at children's hospitals in the United States, 2001-2006. Pediatrics. 2008; 122:1266–1270.

4. Sammons JS, Toltzis P, Zaoutis TE. Clostridium difficile infection in children. JAMA Pediatr. 2013; 167:567–573.

6. Rousseau C, Poilane I, De Pontual L, Maherault AC, Le Monnier A, Collignon A. Clostridium difficile carriage in healthy infants in the community: a potential reservoir for pathogenic strains. Clin Infect Dis. 2012; 55:1209–1215.

7. Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994; 330:257–262.

8. Schutze GE, Willoughby RE. Clostridium difficile infection in infants and children. Pediatrics. 2013; 131:196–200.

9. Sandora TJ, Fung M, Flaherty K, Helsing L, Scanlon P, Potter-Bynoe G, et al. Epidemiology and risk factors for Clostridium difficile infection in children. Pediatr Infect Dis J. 2011; 30:580–584.

10. Rasquin-Weber A, Hyman PE, Cucchiara S, Fleisher DR, Hyams JS, Milla PJ, et al. Childhood functional gastrointestinal disorders. Gut. 1999; 45:Suppl 2. II60–II68.

11. Almeida R, Gerbaba T, Petrof EO. Recurrent Clostridium difficile infection and the microbiome. J Gastroenterol. 2016; 51:1–10.

12. Rineh A, Kelso MJ, Vatansever F, Tegos GP, Hamblin MR. Clostridium difficile infection: molecular pathogenesis and novel therapeutics. Expert Rev Anti Infect Ther. 2014; 12:131–150.

13. Rousseau C, Levenez F, Fouqueray C, Doré J, Collignon A, Lepage P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol. 2011; 49:858–865.

14. Benson L, Song X, Campos J, Singh N. Changing epidemiology of Clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol. 2007; 28:1233–1235.

15. Cohen MB. Clostridium difficile infections: emerging epidemiology and new treatments. J Pediatr Gastroenterol Nutr. 2009; 48:Suppl 2. S63–S65.

16. Owens RC Jr, Donskey CJ, Gaynes RP, Loo VG, Muto CA. Antimicrobial-associated risk factors for Clostridium difficile infection. Clin Infect Dis. 2008; 46:Suppl 1. S19–S31.
17. Benno Y, Sawada K, Mitsuoka T. The intestinal microflora of infants: composition of fecal flora in breast-fed and bottle-fed infants. Microbiol Immunol. 1984; 28:975–986.

18. Rolfe RD, Song W. Immunoglobulin and non-immunoglobulin components of human milk inhibit Clostridium difficile toxin A-receptor binding. J Med Microbiol. 1995; 42:10–19.

19. Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010; 90:859–904.

20. Ang L, Arboleya S, Lihua G, Chuihui Y, Nan Q, Suarez M, et al. The establishment of the infant intestinal microbiome is not affected by rotavirus vaccination. Sci Rep. 2014; 4:7417.

21. Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007; 102:2047–2056. quiz 2057.

22. Mezoff EA, Cohen MB. Acid suppression and the risk of Clostridium difficile infection. J Pediatr. 2013; 163:627–630.

23. Dial S, Delaney JA, Barkun AN, Suissa S. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005; 294:2989–2995.

24. Evans CT, Johnson S. Prevention of Clostridium difficile infection with probiotics. Clin Infect Dis. 2015; 60:Suppl 2. S122–S128.

25. Johnston BC, Ma SS, Goldenberg JZ, Thorlund K, Vandvik PO, Loeb M, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012; 157:878–888.

26. Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013; 382:1249–1257.

27. Na JY, Park JM, Lee KS, Kang JO, Oh SH, Kim YJ. Clinical characteristics of symptomatic Clostridium difficile infection in children: conditions as infection risks and whether probiotics is effective. Pediatr Gastroenterol Hepatol Nutr. 2014; 17:232–238.

28. Pant C, Deshpande A, Altaf MA, Minocha A, Sferra TJ. Clostridium difficile infection in children: a comprehensive review. Curr Med Res Opin. 2013; 29:967–984.
29. Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011; 365:1693–1703.

30. Hung YP, Lee JC, Lin HJ, Liu HC, Wu YH, Tsai PJ, et al. Clinical impact of Clostridium difficile colonization. J Microbiol Immunol Infect. 2015; 48:241–248.

31. Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol. 2006; 101:1894–1899.

32. Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2007; 26:535–544.

33. Lembo A, Pimentel M, Rao SS, Schoenfeld P, Cash B, Weinstock LB, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology. 2016; pii: S0016-5085(16)34926-5. DOI: 10.1053/j.gastro.2016.08.003. [Epub ahead of print].

34. Pérez-Cobas AE, Artacho A, Ott SJ, Moya A, Gosalbes MJ, Latorre A. Structural and functional changes in the gut microbiota associated to Clostridium difficile infection. Front Microbiol. 2014; 5:335.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download