Abstract
Purpose
Blunt trauma of pancreas in children is uncommon and its management varies from observational to early operative intervention. We analysed the feasibility and outcome of non-operative management in all grades of paediatric pancreatic injuries.
Methods
A total of 15 patients of pancreatic trauma seen in a Paediatric Surgery Unit were retrospectively analyzed.
Results
Age of the patients ranged from 3–11 years (mean, 7.7 years). The mode of injury was local trauma in 9 children. Only 3 patients had associated injuries and all were haemodynamically stable. Serum amylase levels were raised in 12 patients at admission which ranged from 400–1,000 IU. Computed tomography scan made a correct diagnosis in 14 patients. Grades of the injury varied from grade I–V (1, 3, 6, 4, 1 patients respectively). Fourteen patients were managed conservatively. One patient underwent laparotomy for suspected superior mesenteric hematoma. The average duration of enteral feeds was 3.7 days and of hospital stay was 9.4 days. Six patients formed pancreatic pseudocysts; two were managed conservatively while the other four underwent cystogastrostomy. The patients were followed up for a period of 1–12 years. All remained asymptomatic and none had exocrine or endocrine deficiencies.
Conclusion
Non-operative treatment for isolated blunt trauma of pancreas in children may be safely followed for all the grades of injury; if associated injuries requiring surgical intervention are ruled out with a good quality imaging and the patients are hemodynamically stable. It did not increase the hospital stay and morbidity and avoided operative intervention on acutely injured pancreas.
Blunt abdominal trauma in Paediatric age group is significant cause of morbidity and mortality [1]. Although pancreatic injury rarely occurs, it is still the fourth most common cause of solid organ injury in paediatric population constituting 3-12% of all blunt abdominal traumas [2]. Non-operative management (NOM) of blunt abdominal trauma involving solid organs has been a standard of care for many years but there is a divided opinion as far as the management of pancreatic trauma is concerned [3]. Early operative intervention is recommended by many as they feel that it leads to decreased incidence of pancreatic complications, however,may not necessarily reduce the length of hospital stay [456].
On the other hand, many other proponents recommend NOM for majority of the pancreatic injuries and argue that only 10% of the patients need secondary surgery. Pseudocyst formation, which is a significant accompaniment of NOM, may form in 50% of the patients but half of them can still be managed non-operatively [78].
Cigdem et al. [7] further advocate expectant management for all grades of blunt trauma of pancreas unless there is haemodynamic instability or associated hollow viscous injury. Hence the optimal management strategy for children with a pancreatic injury still remains to be determined. We retrospectively reviewed the outcome of cases of pancreatic injury managed non-operatively in our unit.
From January 2000 to January 2017, 15 patients of pancreatic injuries were seen in a Paediatric Surgery Unit. The case records were retrospectively analysed and the patients were called for follow up. The study was approved by the Post Graduate Institute of Medical Sciences and Research (IRB no. Ped Surg 1029).
Age of the patients ranged from 3 years to 11 years (mean, 7.7 years). There were 12 males and 3 females. The mode of injury was local trauma such as cycle bar handle (6), cricket bat (1), localised impact of a wooden rod (1) and abdominal punch by a peer (1); other 6 had road traffic accidents (RTA) and fall from the height. Five patients with RTA were referred to Department of Paediatric Surgery, Post Graduate Institute of Medical Education and Research after preliminary resuscitation, whereas, rest of the children presented primarily to us within 8 hours of injury. All the patients were hemodynamically stable at the time of presentation. After initial clinical examination and ultrasound imaging, a pancreatic injury was suspected in 14 out of 15 patients. The samples were drawn for routine haematological and biochemical parameters including serum amylase levels in all the patients. Serum amylase was raised in 12 patients at admission ranging from 400–1,000 IU. All the patients underwent preliminary ultrasonography (USG) which showed free abdominal fluid in all the cases and 2 patients were having liver injury as well (Table 1).
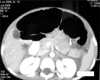
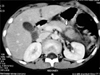
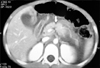
All the patients underwent computed tomography (CT) scan which correctly identified pancreatic injuries in 14 patients. One patient had bilateral pleural effusion as well. Three patients had associated injuries of other organ systems which were grade-II liver tear (2) and head injury (1). The grades of pancreatic injury based on CT imaging, varied from grade I–V as has been described by American Association of Surgery for Trauma grading for pancreatic injuries (Fig. 1, 2, 3, Table 2) [9].
All the patients were started on NOM in form of nasogastric tube placement, intravenous fluids, antibiotics, serial clinical examinations. Based on the clinical examination, hemodynamic stability and the findings of CT imaging, 14 patients were managed conservatively. Out of these 14 patients, one patient had peritoneal tube insertion for drainage of pancreatic ascites due to increasing abdominal distension, which could be removed after 5 days. The other patient, who had pleural effusion, underwent needle aspiration of the fluid from the left hemithorax.
Only one patient was subjected to laparotomy, in whom, the CT scan diagnosed the presence of a superior mesenteric hematoma, threatening the circulation of bowel. On exploration, the patient was found to have a hematoma in the region of head of the pancreas, which was evacuated and the peritoneal cavity drained.
The oral feeds in small increments could be instituted after an average of 3.7 days (range, 3–5 days) in all the patients. The guide for instituting the oral feeds were non-bilious nasogastric aspirates, improved clinical signs in form of softer, pain free abdomen and return of the bowel activity. Eight patients were given partial parenteral nutrition as well for a period ranging from 3 to 5 days. Total hospital stay was 5 to 15 days (mean, 9.4 days) and there was no mortality.
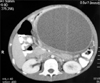
During the follow up, pancreatic pseudocysts had developed in 6 patients from 4–20 weeks after the initial injury (Fig. 4). Out of these 6 patients, 4 underwent cystogastrostomy for increasing size of cyst, epigastic pain, fullness, early satiety, on and off non-bilious vomiting.
The remaining 2 cases had mild epigastric pain with no other symptoms; however, on USG abdomen, they had small cystic collection in lesser sac (diameter of cyst less than 4.5 cm in both the cases). Serial ultrasound assessment, in both the patients, showed a progressive reduction in the size of pseudocyst and a complete resolution in 4 and 5 weeks.
The follow up period ranged from 1–12 years. The patients were growing well and there were no exocrine or endocrine deficiencies.
Blunt trauma of pancreas is uncommon [156] and early diagnosis is difficult as the signs and symptoms are insidious and the imaging modalities, far from accurate. The degree of trauma is also seemingly insignificant as compared to the injuries of liver and spleen. Biochemical parameters like serum amylase and lipase levels may support the clinical suspicion in diagnosis of paediatric pancreatic trauma but have not been found to correlate with severity of the injuries and are of limited value in the management of paediatric pancreatic injuries [9]. Similarly, it may not be cost effective to perform imaging studies based on the serum amylase and lipase levels alone [10]. Serum amylase was raised in 12 of our patients at presentation and helped to strengthen the clinical suspicion of pancreatic injury.
USG is commonly employed as the initial imaging modality in patients of blunt trauma however; the diagnostic accuracy of pancreatic injuries by ultrasound is poor and the grading is based on CT scan images. Although, the CT scan is superior to USG in identifying peripancreatic fluid collections and pancreatic enlargement, but, the ductal injuries still remained poorly identified. In a study by Shilyansky et al. [11] CT scan missed 30% of the significant ductal injuries. Overall, diagnostic accuracy of CT scan in paediatric pancreatic trauma has varied from 69–100% and enhanced accuracy of diagnosis has been associated with lower operative rates [1112]. CT scan is more helpful after 24 hours of injury when the tissue oedema separates the transected edges of the pancreas [513]. In 93.3% (14 out of 15) of our patients, the CT imaging accurately identified the extent of injuries and matched with the clinical picture. Repeated clinical examinations and CT scan were the sheet anchors of NOM in our study.
Magnetic resonance cholangiopancreatography (MRCP) allows direct imaging of the pancreatic duct and sites of its disruption, however; performing a MRCP in a severely injured child has logistic problems [14].
Endoscopic retrograde cholangiopancreatography (ERCP) is safe, accurate and effective diagnostic technique to diagnose the extent of the ductal injury and is used as a guide for early intervention by many [4]. Technical feasibility and the requirement of a general anaesthesia in children are deterrents for its use. Also, the potential complications like pancreatitis, stent migration, and stricture secondary to stent placement need to be considered, which may limit its use in all the cases. Contrast-enhanced CT scan and MRCP should dictate the need for ERCP [15]. Hence interventions which are far from non invasive and carry a significant morbidity of their own become a contentious issue. There is no clarity in literature as to who are the patients who should be subjected to such interventions in order to diagnose ductal injuries, many of which may seem to heal spontaneously. Hence, we subjected all our patients to NOM based on hemodynamic stability and absence of other injuries requiring operative intervention.
Generally, it is considered that grade I-II injuries should be managed conservatively. In a multi-institutional cohort of children with grade II and grade III pancreatic injuries, operative and non-operative strategies appear to have similar outcomes [316]. On-going debate in the literature concerns mainly with the higher grades of pancreatic injuries. The Hospital for Sick Children, Toronto reported 39 patients of pancreatic trauma with grade III or higher, majority (24) were subjected to NOM. However, the 15 patients, who were managed operatively, had a lesser hospital stay and TPN dependency [17]. Early operative therapy (spleen preserving distal pancreatectomy) has been utilised for the treatment of a distal transection of the pancreas. It is associated with lower rates of complications such as pseudocyst formation, readmission, less TPN requirement and shorter hospitalization than NOM [18] Lin et al. [19] reported complication rates ranging from 20% for distal pancreatectomy to above 60% when emergency pancreatic procedures were required in an adult population. Operative management has also been advised for injury to main pancreatic duct (MPD). However, the timely diagnosis of MPD injuries in paediatric patients is a challenge, since as many as 30% of the cases of MPD disruptions are missed even with CT scan. ERCP offers both diagnostic and therapeutic advantage in pancreatic ductal injuries, but its availability and success rate in cannulating the MPD in paediatric age group is still contentious. In cases of partial disruption of duct the procedure of choice is transpapillary pancreatic duct stenting. But in cases where stenting fails, spleen sparing distal pancreatectomy is advised by some authors for distal MPD disruption but is associated with complications like pancreatic fistula, reoperation for small bowel obstruction, wound dehiscence and pseudocyst formation. In disruption of MPD in head region, necrosectomy of head with Roux En Y jejunostomy has been advised [20].
On the other hand, in NOM although there are high chances of pseudocyst formation, but management of pseudocyst is less challenging than any acute surgical intervention [7818].
There may be concerns regarding suitability of NOM in presence of the ductal injuries. Wales et al. [21] reported that NOM was safe and effective in patients with complete pancreatic duct transaction. However, the follow-up abdominal CT scans in 6 out of 8 patients showed a complete atrophy of the body and tail of pancreas but there were no long term exocrine or endocrine deficiencies. In a comparative analysis of 39 surgically managed patients, reported in literature, with 12 conservatively managed patients from personal series by Hamidian Jahromi et al. [22], both operative and non-operative approaches for management of the major ductal injuries were successful with similar complication rates. In a review of the National Pediatric Trauma Registry, the authors reported that early operation for ductal injury without concomitant clinical deterioration may be unwarranted [23].
In our study, hospital stay of the patients subjected to NOM was not significantly more than the patients of similar grade subjected to operative intervention in other studies. Hemodynamic stability of our patients allowed us to proceed with the NOM. Absolute rest to the bowel with active aspiration of the gastric fluids may minimise the stimulus and dry up the secretions of the pancreas as well. This should hasten the process of healing of the pancreatic tissue including the ductal injuries.
The occurrence of the pseudocyst is the main concern following pancreatic trauma managed non-operatively besides concerns about the increased length of hospital stay and enhanced days of parenteral nutrition. In a study, 45% of the traumatic pancreatic pseudocyst required surgical intervention in comparison to 92% of the nontraumatic pancreatic pseudocyst patients [24]. Despite formation of pseudocysts, conservative management has been thought to be the best option by many authors as the subsequent drainage of a pseudocyst is less radical compared to the laparotomies in acutely injured state [25].
In our study, pseudocyst formation was seen in 40.0% (6 out of 15) of the patients with in a period of 4-20 weeks. However, only 26.7% (4 out of 15 patients) needed surgical intervention in form of cystogastrostomy during the follow up. Other authors have reported the pseudocyst formation in 35% of the patients on NOM as compared to 15% in surgically managed patients. The decision of managing a case of pancreatic pseudocyst using conservative or surgical techniques was guided mainly by the severity of patient's symptoms.
Overall, a trend towards non-operative therapy of pancreatic injuries without concomitant increase in morbidity or change in outcomes has been noticed in the recent years and can be safely followed for all the grades. Good quality imaging is one of the key components for following the NOM and early enteral autonomy can be achieved using clinical examination as a guide. The surgery should be reserved for hemodynamically unstable or the ones with hollow organ injuries.
References
1. Jacombs AS, Wines M, Holland A, Ross FI, Shun A, Cass DT. Pancreatic trauma in children. J Pediatr Surg. 2004; 39:96–99.

2. Mattix KD, Tataria M, Holmes J, Kristoffersen K, Brown R, Groner J, et al. Pediatric pancreatic trauma: predictors of nonoperative management failure and associated outcomes. J Pediatr Surg. 2007; 42:340–344.

3. Paul MD, Mooney DP. The management of pancreatic injuries in children: operate or observe. J Pediatr Surg. 2011; 46:1140–1143.

4. Wood JH, Partrick DA, Bruny JL, Sauaia A, Moulton SL. Operative vs nonoperative management of blunt pancreatic trauma in children. J Pediatr Surg. 2010; 45:401–406.

5. Meier DE, Coln CD, Hicks BA, Guzzetta PC. Early operation in children with pancreas transection. J Pediatr Surg. 2001; 36:341–344.

6. Snajdauf J, Rygl M, Kalousová J, Kucera A, Petrů O, Pýcha K, et al. Surgical management of major pancreatic injury in children. Eur J Pediatr Surg. 2007; 17:317–321.

7. Cigdem MK, Senturk S, Onen A, Siga M, Akay H, Otcu S. Nonoperative management of pancreatic injuries in pediatric patients. Surg Today. 2011; 41:655–659.

8. de Blaauw I, Winkelhorst JT, Rieu PN, van der Staak FH, Wijnen MH, Severijnen RS, et al. Pancreatic injury in children: good outcome of nonoperative treatment. J Pediatr Surg. 2008; 43:1640–1643.

9. Herman R, Guire KE, Burd RS, Mooney DP, Ehlrich PF. Utility of amylase and lipase as predictors of grade of injury or outcomes in pediatric patients with pancreatic trauma. J Pediatr Surg. 2011; 46:923–926.

10. Adamson WT, Hebra A, Thomas PB, Wagstaff P, Tagge EP, Othersen HB. Serum amylase and lipase alone are not cost-effective screening methods for pediatric pancreatic trauma. J Pediatr Surg. 2003; 38:354–357. discussion 354-7.

11. Shilyansky J, Sena LM, Kreller M, Chait P, Babyn PS, Filler RM, et al. Nonoperative management of pancreatic injuries in children. J Pediatr Surg. 1998; 33:343–349.

12. Rekhi S, Anderson SW, Rhea JT, Soto JA. Imaging of blunt pancreatic trauma. Emerg Radiol. 2010; 17:13–19.

13. Canty TG Sr, Weinman D. Management of major pancreatic duct injuries in children. J Trauma. 2001; 50:1001–1007.

14. Gupta A, Stuhlfaut JW, Fleming KW, Lucey BC, Soto JA. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics. 2004; 24:1381–1395.

15. Houben CH, Ade-Ajayi N, Patel S, Kane P, Karani J, Devlin J, et al. Traumatic pancreatic duct injury in children: minimally invasive approach to management. J Pediatr Surg. 2007; 42:629–635.

16. Cuenca AG, Islam S. Pediatric pancreatic trauma: trending toward nonoperative management? Am Surg. 2012; 78:1204–1210.

17. Beres AL, Wales PW, Christison-Lagay ER, McClure ME, Fallat ME, Brindle ME. Non-operative management of high-grade pancreatic trauma: is it worth the wait. J Pediatr Surg. 2013; 48:1060–1064.

18. Jobst MA, Canty TG Sr, Lynch FP. Management of pancreatic injury in pediatric blunt abdominal trauma. J Pediatr Surg. 1999; 34:818–823. discussion 823-4.

19. Lin BC, Chen RJ, Fang JF, Hsu YP, Kao YC, Kao JL. Management of blunt major pancreatic injury. J Trauma. 2004; 56:774–778.

20. Fisher M, Brasel K. Evolving management of pancreatic injury. Curr Opin Crit Care. 2011; 17:613–617.

21. Wales PW, Shuckett B, Kim PC. Long-term outcome after nonoperative management of complete traumatic pancreatic transection in children. J Pediatr Surg. 2001; 36:823–827.

22. Hamidian Jahromi A, D'Agostino HR, Zibari GB, Chu QD, Clark C, Shokouh-Amiri H. Surgical versus nonsurgical management of traumatic major pancreatic duct transection: institutional experience and review of the literature. Pancreas. 2013; 42:76–87.
23. Keller MS, Stafford PW, Vane DW. Conservative management of pancreatic trauma in children. J Trauma. 1997; 42:1097–1100.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download