Abstract
Purpose
Celiac disease, an autoimmune enteropathy triggered by exposure to gluten, is not uncommon in South Jordan. However, its prevalence is underestimated due to lack of physician awareness of the diversity of disease presentation. The clinical spectrum includes classic gastrointestinal manifestations, as well as rickets, iron-deficiency anemia, short stature, elevated liver enzymes, and edema. Our goal was to evaluate celiac disease presentation in clinically diagnosed children.
Methods
Retrospective study included all children diagnosed with celiac disease between September 2009 and September 2015. Hospital charts were reviewed. Demographic data, clinical characteristics, and follow-up were recorded.
Results
Thirty-five children were diagnosed with celiac disease during the study period. Mean age±standard deviation was 6.7±3.8 years (range, 2.0–14 years). There were 17 (48.6%) female patients. The average duration between onset of symptoms and diagnosis was 16.3±18.7 months. Fifteen (42.9%) patients presented with classic malabsorption symptoms, whereas 7 (20.0%) patients presented with short stature. Positive tissue transglutaminase antibodies (tTg)-immunoglobulin A (IgA) was seen in 34 (97.1%) patients. The one patient with negative tTg-IgA had IgA deficiency. Although tTG-IgA values were not available for objective documentation of compliance, clinical data (resolution of presenting abnormalities and growth improvement) assured acceptable compliance in 22 (62.9%) patients.
Conclusion
CD in children may present with diverse picture. Although of the small number, the non-classical presentations are not uncommon in our rural community. Gluten-free diet is the main strategy for treatment and associated with usually correction of laboratory abnormalities and improvement of growth.
Celiac disease is an autoimmune enteropathy triggered by exposure to gluten, a complex storage polypeptide in specific grains (wheat, rye, and barley). Gluten consists of multiple distinct proteins, mainly gliadin and glutenin. In genetically susceptible hosts, exposure to gluten-containing food triggers a specific immune response to the gliadin fraction that produces mucosal inflammation, villous atrophy, and increased gut permeability [1].
Celiac disease presents with a wide range of gastrointestinal and extraintestinal manifestations [2]. The disease may present with gastrointestinal manifestations (diarrhea, abdominal bloating, weight loss, vomiting, constipation, and anorexia) within weeks to months of gluten exposure, or it can present with extraintestinal manifestations (chronic fatigue, anemia, osteoporosis, aphthous stomatitis, elevated liver enzymes, joint/muscle pain, infertility, epilepsy, and peripheral neuropathy) [23]. Observation of a strict lifelong gluten-free diet (GFD) is the only effective treatment. Such a diet is expected to resolve the intestinal manifestations and, in most cases, the clinical manifestations [4].
The true prevalence of the disease in our area of the world is underestimated due to the lack of awareness of the atypical presentation of the disease [5]. A previous study from Jordan reported an incidence of celiac disease of 1 in 2,800 live births, with estimated point prevalence of 7:100,000 [6]. Another study estimated the serological prevalence of celiac disease in Jordanian school children to be 1:124 (0.8%; 95% confidence interval, 0.5% to 1.3%) [7].
Celiac disease is not uncommon in Jordan; however, there is little data about the disease characteristics in Jordanian children. A previous case series from our facility documented variability of presentations in our children [8]. In the present study, we aimed to evaluate celiac disease presentation in clinically diagnosed children in South Jordan.
This retrospective study involved all children diagnosed with celiac disease between September 1, 2009 and September 1, 2015. Patients who matched the following criteria were diagnosed with celiac disease: 1) Positive celiac serology and consistent small intestinal biopsy, 2) Classic presentation with 10-fold elevation of tissue transglutaminase antibodies-immunoglobulin A (tTG-IgA) and anti-endomysial antibodies (EMA-IgA) (in which case small-intestine biopsies can be omitted).
The clinical files of patients were reviewed and data extracted regarding age, gender, clinical presentation, laboratory tests, growth parameters, improvement of manifestations, treatment, and compliance to a GFD.
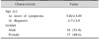
Thirty-five children were diagnosed with celiac disease during the study period. Mean age±SD was 6.7±3.8 years (range, 2.0–14 years). There were 17 (48.6%) female patients (Table 1). The average duration between onset of symptoms and diagnosis was 16.3±18.7 months. Fifteen (42.9%) patients presented with classic malabsorption symptoms, whereas 7 (20.0%) patients presented with short stature.
Celiac disease was diagnosed in two patients with type 1 diabetes, whereas type 1 diabetes developed in one patient 1 year after celiac disease diagnosis. Geophagia was the presenting complaint in two patients. One patient was referred by an ophthalmologist after presenting with xerophthalmia secondary to IgA deficiency.
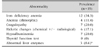
Two patients presented with celiac crises (severe diarrhea, severe hypokalemia, and severe acidosis). Almost half of the patients (16, 45.7%) had anemia. Twelve (34.3%) patients had iron-deficiency anemia, and 4 (11.4%) patients showed mixed dimorphic type of anemia. Coagulopathy and hypoalbuminemia were seen in 7 (20.0%) patients (Table 2) [6]. Prevalence of abnormal blood tests on presentation are shown on Table 3.
Positive tTg-IgA was seen in 34 (97.1%) patients. The one patient with negative tTg-IgA had IgA deficiency.
One patient on GFD developed obesity and elevated lipid profile. Although tTG-IgA values were not available for objective documentation of compliance, clinical data (resolution of presenting abnormalities and growth improvement) assured acceptable compliance in 22 (62.9%) patients.
To our knowledge, this is the first study from South Jordan describing the clinical characteristics of celiac disease in children. The clinical spectrum includes classic gastrointestinal presentations such as chronic diarrhea, abdominal distention, and failure to thrive, as well as rickets, iron-deficiency anemia, short stature, elevated liver enzymes, and edema.
Mean age of onset of symptoms in our study was 5.02±3.09 years, with slight male preponderance. In our cohort, children were older than previously reported in a study from North Jordan (4.6 years). The average age at diagnosis was younger (6.7 vs. 8.4 years). The duration of symptoms prior to diagnosis ranged from 1 month to 11 years. This may reflect the lack of awareness of pediatricians and referring physicians of the diversity of presentation of celiac disease, especially in older children [6].
Male predominance contradicts the well-known female predominance of celiac disease. Previous reports from North Jordan also documented female predominance. Still, male predominance was reported in Pakistani children and in children in some areas of the Russian federation [910].
The gold standard for diagnosing celiac disease is biopsy of the small intestine. Our findings challenge the need for intestinal biopsy with classic presentation and positive serology. High positivity of the serological marker tTG-IgA was highly correlated with MARSH III histopathological changes [111213].
The diagnostic guidelines of The European Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) omit the need for duodenal biopsy in case of classic presentation and highly positive serology testing (tTG-IgA and EMA-IgA) on two separate occasions [11]. Hussain et al. [9] concluded that, with classic presentation of celiac disease and strongly positive tTg-IgA, GFD trial is reasonable when pediatric endoscopy is not readily available. In our cohort, intestinal biopsies were omitted in 12 (34.3%) patients and all responded to GFD.
Celiac crisis is an acute life-threatening condition manifested by severe diarrhea, hypoproteinemia, and metabolic and electrolyte disturbances significant enough to require hospitalization [14]. Two patients in our small series presented with severe watery diarrhea, acidosis, significant electrolyte imbalance, and clotting failure and were diagnosed as celiac crises. Both patients required intensive care unit admission due to severe dehydration and complications of severe hypoelectrolytemia. One of the two patients developed cardiac arrhythmia and paraplegia. Both patients responded to supportive measures, required no steroid treatment, and subsequently responded very well to GFD.
Specific populations are at increased risk of developing celiac disease. Patients with autoimmune diseases, specifically type 1 diabetes, are at increased risk of developing the disease [15]. Celiac disease affects at least 10% of patients with type 1 diabetes at some point in their lives [16], and most are asymptomatic. Therefore, children affected by type 1 diabetes must be screened for celiac disease.
In our cohort 3 (8.6%) patients were selected through screening of asymptomatic diabetics. Interestingly, one of our patients developed diabetes one year after diagnosis of celiac disease. Whereas GFD appears to treat the symptoms and prevent the complications of celiac disease, it will not modify the inherent risk of developing autoimmune diseases.
Having a family member with celiac disease significantly increases the chance of celiac disease in other family members. An estimated pooled prevalence in first-degree relatives is 7.5%, which varies according to the relationship, gender, and geographic location [17]. Unfortunately, due to limited funding we were unable screen asymptomatic family members. Two families have two siblings with celiac disease. The two sets of siblings show similar presentation: failure to thrive, abdominal distention, elevated liver enzymes, and edema.
Abnormal laboratory tests are not uncommon in patients with celiac disease at presentation, and hematological abnormalities are the most prevalent. A previous study from Royal Medical Services reported 30% of their cohort to have anemia [18]. In our cohort anemia was the most prominent abnormality, affecting almost half of the patients (45.7%). Our rates are lower than those reported from the North Jordan cohort, where anemia was reported to affect 70% of the children [6].
GFD is the treatment of choice for celiac disease. Compliance with GFD is difficult to achieve and costly to the patient. It is also difficult to monitor [19]. Rates of compliance with GFD vary from 45% to 81% in children, as reported by the North American Society of Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) [20]. In our cohort, we used improvement of presenting symptoms as a surrogate marker of compliance with GFD. In asymptomatic patients direct inquiry was used to assess compliance. In our cohort, compliance was estimated to be around 63%. This estimate might be inflated by the fact that it depends on subjective assessment only. The noncompliant families reported the lack of availability of affordable alternatives to gluten-containing food as the main cause of noncompliance. In contrast to previously reported improvement of lipid profile and increase in HDL level in celiacs adherent to GFD [21], replacement of gluten-containing food by the family of one patient with a fat-enriched diet led to development of obesity and hyperlipidemia. This highlights the importance of involving a dietician with expertise in GFD to prevent such complications.
In conclusion, celiac disease is not uncommon in South Jordan. Clinical presentation is variable. Duration of symptoms prior to diagnosis remains long. Increased awareness of pediatricians and primary care physicians will improve the detection rate, reduce patient suffering, and decrease morbidity. Affordable GFD appears to improve patient compliance.
Figures and Tables
Table 2
Presenting Features with Comparison to North Jordan

Values are presented as number (%).
*Data from Rawashdeh et al. (J Pediatr Gastroenterol Nutr 1996;23:415-8) with permission [6].
References
2. Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr. 2014; 168:272–278.
3. Ludvigsson JF, Leffler DA, Bai JC, Biagi F, Fasano A, Green PH, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013; 62:43–52.

5. Barada K, Bitar A, Mokadem MA, Hashash JG, Green P. Celiac disease in Middle Eastern and North African countries: a new burden? World J Gastroenterol. 2010; 16:1449–1457.

6. Rawashdeh MO, Khalil B, Raweily E. Celiac disease in Arabs. J Pediatr Gastroenterol Nutr. 1996; 23:415–418.

7. Nusier MK, Brodtkorb HK, Rein SE, Odeh A, Radaideh AM, Klungland H. Serological screening for celiac disease in schoolchildren in Jordan. Is height and weight affected when seropositive? Ital J Pediatr. 2010; 36:16.

8. Altamimi E. Celiac disease in South Jordan: the typical and the atypical. Pediat Therapeut. 2012; DOI: 10.4172/2161-0665.1000134.

9. Hussain S, Sabir MU, Afzal M, Asghar I. Coeliac disease--clinical presentation and diagnosis by anti tissue transglutaminase antibodies titre in children. J Pak Med Assoc. 2014; 64:437–441.
10. Savvateeva LV, Erdes SI, Antishin AS, Zamyatnin AA Jr. Overview of celiac disease in Russia: regional data and estimated prevalence. J Immunol Res. 2017; DOI: 10.1155/2017/2314813. [Epub].

11. Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012; 54:136–160.

12. Hawamdeh H, Al-Zoubi B, AlSharqi Y, Qasrawi A, Abdelaziz Y, Barbar M. Association of tissue transglutaminase antibody titer with duodenal histological changes in children with celiac disease. Gastroenterol Res Pract. 2016; DOI: 10.1155/2016/6718590. [Epub ahead of print].

13. Vivas S, Ruiz de Morales JG, Riestra S, Arias L, Fuentes D, Alvarez N, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastroenterol. 2009; 15:4775–4780.

14. Mones RL, Atienza KV, Youssef NN, Verga B, Mercer GO, Rosh JR. Celiac crisis in the modern era. J Pediatr Gastroenterol Nutr. 2007; 45:480–483.

15. Salardi S, Volta U, Zucchini S, Fiorini E, Maltoni G, Vaira B, et al. Prevalence of celiac disease in children with type 1 diabetes mellitus increased in the mid-1990 s: an 18-year longitudinal study based on anti-endomysial antibodies. J Pediatr Gastroenterol Nutr. 2008; 46:612–614.

16. Rewers M, Liu E, Simmons J, Redondo MJ, Hoffenberg EJ. Celiac disease associated with type 1 diabetes mellitus. Endocrinol Metab Clin North Am. 2004; 33:197–214. xi

17. Singh P, Arora S, Lal S, Strand TA, Makharia GK. Risk of celiac disease in the first- and second-degree relatives of patients with celiac disease: a systematic review and meta-analysis. Am J Gastroenterol. 2015; 110:1539–1548.

18. Rwalah M, Kamal N, Hijazeen R, Ghanma A, Alzeben Z, D'ajeh R. Hematological findings among Jordanian children with celiac disease at presentation: a retrospective analytical study. J R Nav Med Serv. 2014; 21:6–11.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download