Abstract
Purpose
The diagnosis of cow's milk (CM) allergy is a challenge. The Cow's Milk-related-Symptom-Score (CoMiSS™) was developed to offer primary health care providers a reliable diagnostic tool for CM related symptoms. The predictive prospective value of the CoMiSS™ was evaluated in three clinical trials.
Methods
Pooled analyses of the three studies were conducted based on regressing the results of the month-1 challenge test on the month-1 CoMiSS™, adjusting for baseline CoMiSS™ using a logistic regression model. In addition a logistic regression model was also fitted to the month-1 challenge test result with the change in CoMiSS™ from baseline as a predictor.
Results
Results suggest that infants having a low CoMiSS™ (median, 5) after 1 month dietary treatment free from intact CM protein have a significant risk of having a positive challenge test (odds ratio, 0.83; 95% confidence interval, 0.75-0.93; p=0.002). Pooled data suggest that the change in CoMiSS™ from baseline to month-1 can predict CM related symptoms as a confirmed diagnosis according to the challenge test at month-1. However, in order to validate such a tool, infants without CM related symptoms would also need to be enrolled in a validation trial. A concern is that it may not be ethical to expose healthy infants to a therapeutic formula and a challenge test.
Primary health care physicians are insufficiently aware of symptoms caused by cow milk because there is no pathognomonic symptom and no reliable diagnostic test, which results in repeated consultations and inaccurate management. The proposed score has an odds ratio of 0.83 in predicting that an individual patient presenting with cow's milk related symptoms actually has cow's mik allergy, and will therefore increase awareness for cow's milk related symptoms.
Due to the lack of other reliable diagnostic tests other than a food challenge with CM protein, infants suspected of suffering from cow's milk protein allergy (CMPA), developing an awareness tool to recognize cow's milk related symptoms in infants and young children for use by primary healthcare providers may help, as its name implies, in increasing the awareness and the recognition of cow's milk related symptoms. Such a tool, helping in decision-making and correct management, would be appreciated by the parents because it will avoid both over and under-diagnosis, and also shorten the duration of distress of the infants and parental anxiety. Delayed diagnosis of CMPA has a negative impact on the physical development of children [12]. A Cow's Milk-related-Symptom-Score (CoMiSS™), a score that considers general manifestations, dermatological, gastrointestinal and respiratory symptoms, was developed to be used as an awareness tool for cow's milk related symptoms [3] (Table 1). The CoMiSS™ can also be used to evaluate and quantify the evolution of symptoms during a therapeutic intervention. However, the CoMiSS™ does not diagnose CMPA and does not replace a food challenge. Its usefulness needs still to be evaluated by a prospective randomized study.
Three separate clinical trials were conducted to investigate the nutritional adequacy of therapeutic formulas in infants less than 6 months old suspected of having cow's milk related symptoms [456]. Efficacy was measured in terms of the results of a challenge test and the symptom based score, CoMiSS™.
These studies were i) a pilot study conducted by Nestlé Health Science with 116 formula-fed infants between ages 2 weeks and 6 months and suspected of having mild to moderate CMPA [4]; ii) a prospective, randomized, double-blind trial conducted by United Pharmaceuticals/Novalac (Paris, France) with 77 infants of age <6 months and with symptoms suggesting CMPA [5]; and iii) a prospective trial conducted by United Pharmaceuticals/Novalac with 40 infants between the ages of 1 and 6 months with confirmed CMPA via food challenge [6].
In this document, we report the results of a pooled analysis which was conducted to investigate the feasibility of using the CoMiSS™ as a predictor of cow's-milk-related-symptoms suggesting CMPA as determined by an open challenge test with cow's milk based infant formula.
Data from the three studies were selected for the pooled analysis based on the following criteria: i) The challenge test result was available; ii) The CoMiSS™ was completed both at baseline and at one month on an elimination diet (month-1). Pooled analyses were conducted based on regressing the results of the month-1 challenge test on the month-1 CoMiSS™, adjusting for baseline CoMiSS™ using a logistic regression model. In addition, a logistic regression model was also fitted to the month-1 challenge test result with the change in CoMiSS™ from baseline as a predictor. In the original study for which the CoMiSS™ was developed, the cut-off for inclusion in the study was set at >12 [4]. In the second study, the cut-off was set at >10 [5], and in the third study there was no cut-off of the CoMiSS™ for inclusion in the study [6].
A logistic regression model was fitted with month-1 challenge test (positive or negative) as the dependent variable and month-1 CoMiSS™ as the predictor while adjusting for the baseline CoMiSS™. We further adjusted for age, gender and study. Results are summarized in terms of odds ratio (OR) with 95% confidence interval (CI) and p-value. In order to assess the accuracy of prediction, the pooled data was split into training and test sets using different proportions ranging from 50:50 split to a 90:10 split where a logistic regression was fitted using the training data while this model was used to predict the test data. The area under the curve (AUC) of the receiver operating characteristics (ROC) curve was evaluated at each split in order to obtain the learning-curve. The ROC curve is the curve of sensitivity against specificity for all possible thresholds on the month-1 CoMiSS™ (or the change from baseline) as a predictor of the result of the challenge test. The AUC of ROC curves vary between 0.5 to 1 with 0.5 representing random prediction while 1 representing perfect prediction. Values of AUC above 0.8 can lead to useful prediction. The learning curve in general is used to see if and how the prediction improves with increasing training set size and may be useful in sample-size calculations for future trials aiming at validating the CoMiSS™ as a predictor of cow's milk related symptoms such as CMPA as determined by the challenge test. The data used for the above mentioned analysis are listed in Table 2.
The baseline characteristics for the trials were quite similar, with a median age at inclusion around 2.5 months and very similar CoMiSS™ scores, despite the different criteria of CoMiSS™ for inclusion [456].
As also shown in Table 1, the majority of the infants had a lower CoMiSS™ at month-1 (median, 5) compared to baseline (median, 13). While 63% of the infants in the different studies had a CoMiSS™ >12 at baseline, only 2.7 of the infants still had a CoMiSS™ >12 after one month of the elimination diet. In the Nestlé Health Science study, 69% of the infants had a positive challenge test while this was the case in 81.1% infants in the two Novalac studies.
Results suggest that age, gender and study are not confounders to the relationship between the challenge test result and the CoMiSS™.
Infants having a low CoMiSS™ at month-1 after an intact cow's milk protein elimination diet while fed an extensively hydrolyzed formula have a significant risk of having a positive challenge test (OR, 0.83; 95% CI, 0.75-0.93; p=0.002).
A training-test split prediction analysis yielded to an AUC around 0.7 for each of the split ratios. The prediction is less than satisfactory with the current data as the trials only enrolled infants with a high baseline CoMiSS™ and as such the prediction lacks in specificity. In fact, the minimum baseline CoMiSS™ score in the three studies was 6 with the 25 % percentile being 12. The distribution of the CoMiSS™ score is yet to be determined for healthy and symptom-free infants. Numerical studies with synthetic data suggest that if prediction models are built on “all-comers” that cover the entire range of the CoMiSS™ score while assuming a normal distribution, then an AUC for prediction as high as 0.9 can be achieved based on the relationship between the CoMiSS™ and challenge test results as described by a logistic regression model. This is illustrated in Fig. 1 which shows the ROC curve with an AUC of 0.88 for prediction of the challenge test result for new infants given their change in CoMiSS™ score from baseline to month-1 with artificial data generated for healthy and symptom-free infants pooled with the data from the three studies with symptomatic infants at baseline.
The results of the pooled data analysis suggest that the change in CoMiSS™ from baseline to one month on a cow's milk protein free diet can be used to predict cow's-milk-related-symptoms as confirmed using the challenge test at month-1.
There are no similar data available in literature. There is no previous report on a “symptom score” for cow's milk related symptom.
The original findings applying a CoMiSS™ >12 for inclusion as listed in Table 1 [4] are confirmed with the pooled data, using also a CoMiSS™ of ≥10 as inclusion criterion or even no CoMiSS™-value as cut-off for inclusion [56]. However, in order to validate such a tool, infants without cow's milk related symptoms would also need to be enrolled in the validation trial. One obvious concern is that it may not be ethical to expose healthy infants to the challenge test. One possibility is to conduct the CoMiSS™ on such infants at baseline and month-1 and assume that they would have a negative result on the challenge test.
Although insufficiently powered to allow firm conclusions, the findings do suggest that a CoMiSS™ of >12 may be a good cut-off value to select infants presenting symptoms related to cow's milk protein.
In the meantime the CoMiSS™ has been shown to be reliable tool in increasing awareness of primary health care physicians to more accurately suspect cow's-milk-related-symptoms. In order to use the score as a diagnostic tool, a validation trail is still needed.
The results of the pooled analysis confirm that the CoMiSS™ may be a sensitive and specific awareness tool for health care professionals to select infants suspected to present with cow's milk related symptoms.
Figures and Tables
Fig. 1
The receiver operating characteristics (ROC)-curve predicting probability to have positive challenge test. A typical ROC curve for predicting month-1 challenge test result based on the change in Cow's Milk-related-Symptom-Score (CoMiSS™) from baseline to month-1 and based on data generated to cover the entire range of the CoMiSS™ (0-12). The area under the curve (AUC) of the ROC curve is 0.88. The thin straight line depicts random prediction with an AUC of 0.5.
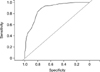
Table 1
The Cow's-Milk-related-Symptom-Score (CoMiSS™)

Although many infants with CoMiSS™ have no impaired growth or weight gain, faltering of these parameters suggests organic disease, of which cow's milk protein allergy is a possible cause.
*Crying was only considered if the child was crying for one week or more, assessed by the parents, without any other obvious cause.
Adapted from Vandenplas et al. (Acta Paediatr 2013;102:990-8), with permission [4].
ACKNOWLEDGEMENTS
Yvan Vandenplas has participated as a clinical investigator, and/or advisory board member, and/or consultant, and/or speaker for Abbott Nutrition, Aspen, Biogaia, Biocodex, Danone, Hero, Nestle Nutrition Institute, Nutricia, Mead Johnson Nutrition, Merck, Orafti, Phacobel, Sari Husada, United Pharmaceuticals, Wyeth and Yakult.
P Steenhout and A Järvi are employees of Nestlé Health Sciences.
AS Garreau is an employee of United Pharmaceuticals.
R. Mukherjee reported no COI.
References
1. Robbins KA, Wood RA, Keet CA. Milk allergy is associated with decreased growth in US children. J Allergy Clin Immunol. 2014; 134:1466–1468.e6.

2. Vieira MC, Morais MB, Spolidoro JV, Toporovski MS, Cardoso AL, Araujo GT, et al. A survey on clinical presentation and nutritional status of infants with suspected cow' milk allergy. BMC Pediatr. 2010; 10:25.

3. Vandenplas Y, Dupont C, Eigenmann P, Host A, Kuitunen M, Ribes-Koninckx C, et al. A workshop report on the development of the cow's milk-related symptom score awareness tool for young children. Acta Paediatr. 2015; 104:334–339.

4. Vandenplas Y, Steenhout P, Planoudis Y, Grathwohl D. Althera Study Group. Treating cow's milk protein allergy: a double-blind randomized trial comparing two extensively hydrolysed formulas with probiotics. Acta Paediatr. 2013; 102:990–998.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download