Abstract
Eosinophilic gastrointestinal disorder (EGID) is a rare disease in children that affects the bowel wall, with eosinophilic infiltration in the absence of any other causes for eosinophilia. The etiology remains unknown, but allergies and immunological imbalance are suspected triggers. We encountered a case of serosal EGID presenting as intractable vomiting and ascites in a 9-year-old girl, after influenza virus infection. Peripheral eosinophilia was not present. The diagnosis was confirmed by biopsy of the bowel wall through laparotomy and endoscopy, and controlled by 2 courses of steroid therapy due to recurring symptoms. Influenza virus infection was assumed to play a role in the onset of EGID through a Th2 response that stimulated eosinophilic infiltration in the GI tract. We therefore report this case along with a literature review.
Eosinophilic gastrointestinal disorders (EGIDs) are uncommon diseases characterized by marked eosinophilic infiltration of the gastrointestinal (GI) tract [1]. There are 3 major types: the mucosal type is the most common, followed by the muscle type, and the rare serosal type [2]. The various clinical manifestations are related to the site and depth in the involved GI tract. However, the pathogenesis remains unclear. Recent reports suggested that EGID may be associated with immunoglobulin E (IgE)-mediated allergy and delayed T-helper type 2 (Th2) response through a variety of triggers [1,3].
Only a few other cases of EGID implicating a viral infection as the trigger have been reported [4,5,6], but there have been no reports of EGID patients after influenza virus infection. Therefore, we report a case of EGID with features of the serosal type confirmed by biopsy, presenting as intractable vomiting and ascites in a girl during the recovery period of influenza A infection.
A 9-year-old girl was admitted to the emergency department complaining of severe, colicky abdominal pain with bilious vomiting and loss of appetite. She had not urinated for 12 hours, and her skin was dehydrated. She had a history of influenza A virus infection diagnosed by reverse transcription polymerase chain reaction (RT-PCR) from a nasopharyngeal specimen 1 week earlier, but no history of abdominal surgery, allergic disease, or food sensitivity. On physical examination, she appeared acutely ill, but alert mentally. Her body temperature was 36.0℃, blood pressure was 122/87 mmHg, pulse was 98/min, and respiratory rate was 22/min. Chest auscultation demonstrated decreased breath sounds over both the lower lobes. Her abdomen was markedly distended and bowel sounds were decreased. Tenderness was evident over the entire abdomen with shifting abdominal dullness, but no rebound tenderness.
Laboratory investigation revealed a white blood cell count of 12,870/mm3, with 93.0% neutrophils and 0% eosinophils, hemoglobin level of 17.1 g/dL, and platelet count of 168×103/mm3. Electrolytes showed sodium levels decreased to 124 mmol/L; potassium, 4.3 mmol/L; and chloride, 81 mmol/L. The protein level was 6.7 g/dL; albumin, 3.9 g/dL; blood urea nitrogen, 59.2 mg/dL; creatinine, 2.08 mg/dL; erythrocyte sedimentation rate, 12 mm/h; and C-reactive protein, 20.2 mg/dL. The stool calprotectin level was increased to 1,383.0 mg/kg. Other data were unremarkable.
A simple chest radiograph showed a small amount of bilateral pleural effusion without an active lung lesion. A simple abdominal radiograph revealed multiple air-fluid levels in the small intestine. Abdominopelvic computed tomography (CT) showed dilatation of the small intestine and ascites with nodular and edematous bowel wall thickening from the distal esophagus to the entire colon (Fig. 1).
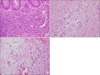
She was treated with intravenous fluid therapy for prerenal azotemia (fractional excretion of sodium, 0.1%) with hyponatremia due to GI loss. An emergency laparotomy was performed for suspected mechanical obstruction. As the peritoneum was opened, a large amount of ascites was seen in the peritoneal cavity. Exploration revealed an edematous and dilated small bowel, including the appendix, but no obstruction was seen. Appendectomy was performed. The resected specimen showed edematous mucosa and swollen mucosal folds. Histologically, diffuse infiltration by eosinophils was observed in the submucosa, muscle, and subserosa (Fig. 2).
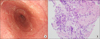
Esophagogastroduodenoscopy (EGD) showed trachealization at the mild-esophagus (Fig. 3A) and edematous duodenal mucosa. Biopsy of the esophagus and duodenum revealed inflammation with increased eosinophilic infiltration in the submucosa and mucosa, respectively (more than 20/high power field) (Fig. 3B). Ascitic and pleural fluid revealed a transudate without eosinophilia, malignant cells, or microorganisms.
Postoperatively, serum total IgE (immunoCAP®; Phadia AB, Uppsala, Sweden) was within normal limits (252 kU/L). Fluorescent antinuclear antibody screening was weakly positive and antineutrophil cytoplasmic antibody screening was negative. Anti-Saccharomyces cerevisiae antibodies, IgG, and IgA were negative. Mycoplasma pneumoniae-PCR testing of sputum was negative. Stool microscopy for ova and parasites was negative.
After excluding malignancy, inflammatory disease, autoimmune disease, and infection, the patient was diagnosed with eosinophilic gastroenteritis and eosinophilic colitis. She was treated with 15 mg/day of prednisolone for 2 weeks, with rapid improvement in symptoms.
After terminating steroid therapy, she again complained of epigastric pain, vomiting, and diarrhea, without peripheral eosinophilia. Abdominal CT showed residual wall thickening from the transverse to descending colon and gastric antrum. Second-look EGD and colonoscopy revealed normal mucosa in the esophagus and colon. Multiple biopsies showed a few eosinophilic infiltrates in the submucosa (up to 20 per high-power field) at the gastric antrum and terminal ileum. She was treated with 40 mg/day prednisolone, and her symptoms resolved. Steroid treatment was gradually tapered and terminated after 5 weeks without need for maintenance treatment. The level of stool calprotectin normalized to 42.1 mg/kg. She was free of recurrence during a follow-up period of 6 months.
The definition of EGID is the presence of GI symptoms, a biopsy demonstrating eosinophilic infiltration of one or more areas of the GI tract, and the absence of parasitic or extraintestinal diseases that could cause eosinophilia such as malignancy, drug reactions, or vasculitis [3]. In our case, the patient's GI symptoms and the findings on abdominal radiography and CT led us to suspect mechanical obstruction, and a full-thickness biopsy of the appendix demonstrated eosinophilic infiltration from the submucosa to the subserosa, in the absence of peripheral eosinophilia. Moreover, endoscopic biopsy of the esophagus and duodenum revealed eosinophilic infiltration in the submucosa and mucosa, respectively. We excluded other disease based on past history, laboratory results, stool tests, radiologic tests, and endoscopy. Thus, the patient was diagnosed with serosal type EGID, extending from the esophagus to the colon. The clinical symptoms improved after treatment with a low dose of steroids. Although the symptoms recurred 2 weeks after treatment cessation, the patient's condition again improved with a higher dose of steroid treatment for 5 weeks.
The pathogenesis of EGID is still unknown, but IgE-mediated allergies and delayed Th2 response are suspected causes [3]. In our case, the characteristics of IgE-mediated allergy were not seen because the patient had no atopic manifestations, and normal levels of IgE. Although little is known about viral infection as a trigger of EGID, studies of eosinophilic infiltration in the GI tract of patients with cytomegalovirus and Epstein-Barr virus infection [45] led to the assumption that influenza virus infection could be associated with EGID. Our patient had been diagnosed with influenza A virus infection by RT-PCR in nasal secretions 1 week previously, and this infection is itself often accompanied by GI symptoms [67]. This case did not meet the criteria for conventional influenza virus infection with GI manifestation for the following reasons: 1) the GI symptoms developed during the recovery period after influenza A infection and 2) local eosinophilic infiltration through the subserosal level was sufficient to diagnosis EGID. We could not be certain of the correlation between the onset of EGID and influenza virus infection, other than by temporal association, because influenza virus was not detected in feces and GI tissues. However, late onset GI symptoms after influenza infection and histologic diagnosis of EGID suggest that EGID is not consistent with the activation of a Th1 response induced by a conventional virus infection [8]. Imbalance in Th1 and Th2 responses may shift according to host immunity and type of virus. There are studies showing that Th2 response can be induced by the same type of influenza infection, depending on the immune status of the host [910]. Another study reported a Th2 response, early induction of interleukin (IL)-5, and eosinophilia caused by 2009 H1N1 infection [11]. Therefore, there is a possibility that influenza A virus infection can induce a Th2 response. Although there is weak evidence that the cause of the EGID was influenza virus infection, we hypothesize that infected immune cells in the GI tract induced a Th2 response that stimulated eosinophilic infiltration in the GI tract.
This disease is classified into 3 types on the basis of the depth of eosinophilic infiltration, as proposed by Klein et al. [2]. Mucosal disease is the most common form, and presents with vomiting, diarrhea, bleeding, malabsorption, and protein-losing enteropathy. Muscular disease causes bowel wall thickening and subsequent bowel obstruction. Serosal disease is rare, and results in peritonitis or ascites, which was the case in this patient. In our case, eosinophils infiltrated all 3 layers of the intestinal wall. Muscle layer involvement caused intestinal wall thickening, with the appearance of obstruction, and subserosal involvement produced transudative ascites. To date, only a few case of serosal type EGID in children have been reported in Korea [121314].
There are limitations in this report. We were not able to detect influenza virus in tissue and feces and did not test for Th2-related interleukins to support our hypothesis.
In summary, our case is a rare serosal type of EGID confirmed by histologic findings in a child during the recovery period after influenza A infection. We cannot conclude that there is an association between influenza virus and EGID. Therefore, further research is needed on the pathogenesis of influenza virus involving the GI tract and acting as a trigger for the development of EGID.
Figures and Tables
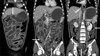 | Fig. 1Abdominopelvic computed tomography showed a large amount of ascites and nodular and edematous wall thickening of small bowel loops (arrows). |
References
2. Klein NC, Hargrove RL, Sleisenger MH, Jeffries GH. Eosinophilic gastroenteritis. Medicine (Baltimore). 1970; 49:299–319.

3. Rothenberg ME. Eosinophilic gastrointestinal disorders (EGID). J Allergy Clin Immunol. 2004; 113:11–28.

4. Takeyama J, Abukawa D, Miura K. Eosinophilic gastroenteritis with cytomegalovirus infection in an immunocompetent child. World J Gastroenterol. 2007; 13:4653–4654.

5. Koga M, Fujiwara M, Hotta N, Matsubara T, Suzuki E, Furukawa S. Eosinophilic gastroenteritis associated with Epstein-Barr virus infection in a young boy. J Pediatr Gastroenterol Nutr. 2001; 33:610–612.

6. Minodier L, Charrel RN, Ceccaldi PE, van der, Blanchon T, Hanslik T, et al. Prevalence of gastrointestinal symptoms in patients with influenza, clinical significance, and pathophysiology of human influenza viruses in faecal samples: what do we know? Virol J. 2015; 12:215.

7. Vivar KL, Uyeki TM. Influenza virus infection mimicking an acute abdomen in a female adolescent. Influenza Other Respir Viruses. 2014; 8:140–141.

8. Wohlleben G, Müller J, Tatsch U, Hambrecht C, Herz U, Renz H, et al. Influenza A virus infection inhibits the efficient recruitment of Th2 cells into the airways and the development of airway eosinophilia. J Immunol. 2003; 170:4601–4611.

9. Kristjansson S, Bjarnarson SP, Wennergren G, Palsdottir AH, Arnadottir T, Haraldsson A, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local Th2-like response. J Allergy Clin Immunol. 2005; 116:805–811.

10. Yu X, Zhang X, Zhao B, Wang J, Zhu Z, Teng Z, et al. Intensive cytokine induction in pandemic H1N1 influenza virus infection accompanied by robust production of IL-10 and IL-6. PLoS One. 2011; 6:e28680 .

11. Terai M, Honda T, Yamamoto S, Yoshida M, Tsuchiya N, Moriyama Y, et al. Early induction of interleukin-5 and peripheral eosinophilia in acute pneumonia in Japanese children infected by pandemic 2009 influenza A in the Tokyo area. Microbiol Immunol. 2011; 55:341–346.

12. Choi JS, Choi SJ, Lee KJ, Kim A, Yoo JK, Yang HR, et al. Clinical manifestations and treatment outcomes of eosinophilic gastroenteritis in children. Pediatr Gastro-Enterol Hepatol Nut. 2015; 18:253–260.

13. Yi ES, Kim MJ, Ha SY, Lee YM, Choi KE, Choe YH. A case of non-IgE-mediated eosinophilic gastroenteritis presenting as ascites. Korean J Pediatr Gastroenterol Nutr. 2011; 14:181–186.

14. Yun KT, Jeon YC, Son YW, Sohn JH, Yoon BC, Choi HS, et al. A case of eosinophilic enteritis with ascites. Korean J Gastroenterol. 2000; 35:507–511.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download