Abstract
Noroviruses have been recognized as the leading cause of epidemic and sporadic gastroenteritis since the advent of molecular diagnostic technique. They have been documented in 5-31% of pediatric patients hospitalized with gastroenteritis. Although norovirus gastroenteritis is typically mild and self-limited, it causes severe, but sometimes fatal, conditions in the vulnerable population such as immunocompromised patients, young children, and the elderly. Bowel perforation due to norovirus infection is rare. We report a case of small bowel perforation with norovirus gastroenteritis in the infant with Down syndrome during the hospitalization with pneumonia. Severe dehydration may cause bowel ischemia and could have triggered bowel perforation in this case. Physicians should be alert to the potential surgical complications followed by severe acute diarrhea, especially in high risk groups.
Noroviruses (NoVs) have been recognized as the leading cause of epidemic and sporadic gastroenteritis since the advent of molecular diagnostic technique. NoVs are a separate genus in the family Caliciviridae and have great diversity of genogroups and genotypes [1]. Most of the strains associated to human disease belong to genetic clusters within genogroups I and II (GI and GII) [1]. According to a recent epidemiologic study, waterborne outbreaks are significantly associated with GI strains, while healthcare-related and winter outbreaks are associated with GII strains [2].
Three-month-old female infant was admitted for the management of fever, cough and dyspnea. She was born at 35 weeks gestation by Cesarian section with birth weight of 2.46 kg. After birth, she was diagnosed as Down syndrome due to a characteristic morphology and chromosomal anomaly. At that time, a small ventricular septal defect was discovered by echocardiogram. Her height was 60 cm (50 percentile) and body weight was 4.05 kg (<3 percentile). Physical examinations of chest revealed crackles and moderate grade of subcostal retraction. Laboratory findings were nonspecific with normal C-reactive protein and mild elevation of liver enzymes (aspartate aminotransferase/alanine aminotransferase 50/81 mg/dL). No virus was detected in the respiraotory virus polymerase chain reaction (PCR) assay in nasopharyngeal aspirates. Her chest x-rays showed bilateral peribronchial infiltrations. She was managed under the diagnosis of viral pneumonia and improved with treatment.
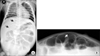
On day 8, high-grade fever, vomiting, and watery diarrhea developed. After 2 days of gastroenteritis symptoms, laboratory findings revealed metabolic acidosis and prerenal failure features due to a dehydration by frequent (20 times a day) diarrhea (pH, 6.90; HCO3-, 7.2 mmol/L; blood urea nitrogen (BUN)/Cr, 49.4/2.3 mg/dL; Na+/K+/Cl-, 138/5.1/117 mmol/L; anion gap, 18.9). At that time, her pulse rate was 210/min, body temperature was 39.9℃ and body weight was 4.0 kg. Her volume status was underestimated and total intake of volume (maintenance fluid volume by 5% dextrose sodium potassium (Na 34, K 20 meq/L, 20 mL/hr) and oral intake) was not adequate for her sudden volume loss over the short period. She was transferred to the intensive care unit for close monitoring and aggressive volume therapy. After delivering a bolus of normal saline (40 mL/kg) and bicarbonate infusion, 5% dextrose in 0.9% normal saline was infused (20 mL/hr). Laboratory findings were normalized (pH, 7.31; HCO3-, 21.0; BUN/Cr, 16.5/0.5 mg/dL) on day 10 and maintenance fluid was changed to 5% Dextrose sodium potassium (Na 34, K 20 meq/L, 20 mL/hr). However, her abdomen was severely distended and plain radiograms of abdomen showed dilatation of the small intestine. We performed abdominal ultrasonography and it showed dilatation and wall thickening of small bowel loops indicating small bowel obstruction. We closely observed her for the possibility of development of acute surgical abdomen. On day 11, plain abdominal radiogram showed suspicious free gas on upper abdomen (Fig. 1A). We performed the crosstable abdominal radiogram and it showed free bowel gas (Fig. 1B). She underwent an emergent laparotomy. Multiple necroses with 1 cm perforation were observed in terminal ileum 10 cm away from the ileocecal valve (Fig. 2). Distension of the whole small bowel and peritonitis were also found. Segmental resection of terminal ileum was performed. Histopathology of the resected bowel revealed a transmural coagulative necrosis, submucosal congestion, and focal lymphoid hyperplasia. Fecal specimen culture was negative but reverse transcriptase-PCR (RT-PCR) for NoV was positive. It was reported as GII and genotype 4 (GII. 4). The antigen test and enzyme immunoassay for adenovirus and rotavirus were negative, respectively. RT-PCR for astrovirus was also negative. Cytomegalovirus workup was not performed. After the operation, her abdominal distension improved and oral feeding was started on the day 9 after operation. However, bacterial pneumonia developed and she was intubated on day 21. We performed laboratory tests for the evaluation of her immune functions. She had a normal B, T lymphocytes and Natural-Killer cell counts. Immunoglobulin G/A/M levels were also within the normal range. She was discharged on day 48 in good general condition.
RT-PCR for the diagnosis of NoV infection has substantially changed understanding of their epidemiology. NoVs has been documented in 5-31% of pediatric patients hospitalized with gastroenteritis and perhaps the second most common cause of diarrhea leading to hospitalization of children aged <5 years old [1]. In developing countries, NoVs are suggested as the cause of severe illness and death (218,000 deaths each year) from diarrhea among children [4].
Recently, the most common sequence of NoV has been GII. 4 genotype [156]. In the present report, the strain of NoV was GII. 4. The rapid evolution of GII. 4 variants had led to the emergence of novel strains associated with global epidemics and nosocomial outbreaks [56]. Frequent nosocomial transmission of NoV infection has been reported and been an economic burden in healthcare settings [78]. In the present case, NoV gastroenteritis was presumed to be a nosocomial infection. It developed on day 8 of the hospitalization and the patient had no contact with the individuals with gastroenteritis symptoms before her admission. One recent study demonstrates frequent missed cases of NoV infections despite laboratory testing and underdiagnosis of NoV patients are associated with costly abdominal imaging and nosocomial clustering [9].
Bowel perforation due to NoV gastroenteritis is rare. In adults, only one case of spontaneous bowel perforation due to NoV infection was reported in an 83 year old Caucasian patient [10]. In the previous case, necrosis with a perforation was observed in mid-ileum. Similarly necrotic lesions with a perforation were in the terminal ileum in our case. It is suggested that histologic changes of NoV infection such as a broadening and blunting of the intestinal villi, crypt-cell hyperplasia, cytoplasmic vacuolization, and infiltration of inflammatory cells into the lamina propria were more prominent in proximal small intestine [111]. In preterm babies, association of NoV infection with necrotizing enterocolitis (NEC) were reported [1213]. Severe dehydration with acute diarrhea may cause bowel ischemia and lead to perforation [1415]. Similarly in our case, severe dehydration could have triggered ileal perforation. Pathologic findings of the present case showed transmural coagulative necrosis and this suggests bowel ischemia, which is characteristic of NEC [16]. Furthermore, we speculated severe symptoms of gastroenteritis in the present patient were partly due to possible intrinsic or functional defect of immune function known in Down syndrome [17]. However, the performed tests for immune functions showed no definite abnormalities overall. It has been suggested that children with Down syndrome have intrinsic defect in B lymphocytes besides the apparent thymus and T lymphocytes abnormalities [18]. In immunocompromised patients, young children, and the elderly, severe or chronic disease of NoV infection can occur [4].
In conclusion, we report bowel perforation with severe NoV gastroenteritis developed in 3-month-old infant with Down syndrome. NoVs can cause severe gastroenteritis and fatal complications. Therefore, physicians should be alert to the potential surgical complications followed by severe acute diarrhea, especially in high risk groups.
Figures and Tables
References
2. Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, et al. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. 2012; 140:1161–1172.

3. Mattner F, Sohr D, Heim A, Gastmeier P, Vennema H, Koopmans M. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin Microbiol Infect. 2006; 12:69–74.

4. Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinjé J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008; 14:1224–1231.

5. Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. 2006; 44:327–333.

6. Mans J, de Villiers JC, du Plessis NM, Avenant T, Taylor MB. Emerging norovirus GII.4 2008 variant detected in hospitalised paediatric patients in South Africa. J Clin Virol. 2010; 49:258–264.

7. Johnston CP, Qiu H, Ticehurst JR, Dickson C, Rosenbaum P, Lawson P, et al. Outbreak management and implications of a nosocomial norovirus outbreak. Clin Infect Dis. 2007; 45:534–540.

8. Beersma MF, Schutten M, Vennema H, Hartwig NG, Mes TH, Osterhaus AD, et al. Norovirus in a Dutch tertiary care hospital (2002-2007): frequent nosocomial transmission and dominance of GIIb strains in young children. J Hosp Infect. 2009; 71:199–205.

9. Beersma MF, Sukhrie FH, Bogerman J, Verhoef L, Mde Melo M, Vonk AG, et al. Unrecognized norovirus infections in health care institutions and their clinical impact. J Clin Microbiol. 2012; 50:3040–3045.

10. Pawa N, Vanezis AP, Tutton MG. Spontaneous bowel perforation due to norovirus: a case report. Cases J. 2009; 2:9101.

11. Souza M, Azevedo MS, Jung K, Cheetham S, Saif LJ. Pathogenesis and immune responses in gnotobiotic calves after infection with the genogroup II.4-HS66 strain of human norovirus. J Virol. 2008; 82:1777–1786.

12. Pelizzo G, Nakib G, Goruppi I, Fusillo M, Scorletti F, Mencherini S, et al. Isolated colon ischemia with norovirus infection in preterm babies: a case series. J Med Case Rep. 2013; 7:108.

13. Turcios-Ruiz RM, Axelrod P, St John K, Bullitt E, Donahue J, Robinson N, et al. Outbreak of necrotizing enterocolitis caused by norovirus in a neonatal intensive care unit. J Pediatr. 2008; 153:339–344.

14. Ueda N, Shimotake T, Ohama K. Duodenal perforation associated with norovirus and rotavirus gastroenteritis. Clin Case Rep. 2013; 1:47–49.

15. Chen JC, Chen CC, Liang JT, Huang SF. Spontaneous bowel perforation in infants and young children: a clinicopathologic analysis of pathogenesis. J Pediatr Gastroenterol Nutr. 2000; 30:432–435.

16. Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten-year experience. J Pediatr. 1990; 117:S6–S13.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download