Abstract
Mycoplasma pneumoniae infections mainly involve respiratory tract; however, also can manifestate other symptoms by site involved. Extrapulmonary manifestations of M. pneumoniae infection are rarely known to occur without pneumonia. Herein we report a case of a 9-year-old boy who presented with acute cholestatic hepatitis in the absence of pneumonia. Rhabdomyolysis, skin rash, and initial laboratory results suspicious of disseminated intravascular coagulopathy were also observed in this patient. M. pneumoniae infection was identified by a 4-fold increase in immunoglobulin G antibodies to M. pneumoniae between acute and convalescent sera by enzyme-linked immunosorbent assay. This is the first pediatric case in Korea of M. pneumoniae infection presenting with acute cholestatic hepatitis in the absence of pneumonia.
Mycoplasma pneumoniae is a major cause of respiratory infection in school-age children and adolescents. Extrapulmonary manifestations of M. pneumoniae infection are known to affect dermatologic, cardiovascular, neurologic, hematologic and hepatobiliary systems in children, which are usually known to occur as complications after pneumonia [1]. However, acute cholestatic hepatitis without pulmonary involvement in children with M. pneumonia infection has rarely been reported [23]. We report a rare case of a child with M. pneumonia infection presenting with acute cholestatic hepatitis and other extrapulmonary manifestations in the absence of pneumonia.
A 9-year-old boy was admitted to our hospital due to fever and abdominal pain, which had developed 4 days before. Ten days prior to admission, the patient had developed mild cough and body temperature elevation up to 37.6℃, which improved within 2 days without treatment. However, he newly developed fever and right upper quadrant pain 4 days later. Symptoms aggravated and the patient visited our hospital. Past medical history of the patient and family were both unremarkable. Vaccination had been performed as scheduled. No recent history of travel or trauma was reported.
On admission, he was fully conscious and oriented. Vital signs showed a blood pressure of 88/51 mmHg, heart rate of 97 beats/min, respiratory rate of 27 breaths/min, and body temperature of 38.7℃. His heart beat was regular without murmurs, and breath sounds were clear on both lung fields. His abdomen was soft and flat with normoactive bowel sounds. However, there was tenderness in the right upper quadrant region and hepatomegaly of 3 fingerbreadths and splenomegaly of 1 fingerbreadth were palpated below the costal margins. Erythematous maculopapular skin rashes without pruritis were observed on both lower extremities. Neurologic examination was normal.
Initial laboratory exams showed a hemoglobin of 13.1 g/dL, hematocrit 37.0%, white blood cell (WBC) count of 6,310/mm3 with 72% neutrophils, 11% lymphocytes, 2% monocytes, and platelet count of 89,000/mm3. Chemistry exams revealed an elevated C-reactive protein (CRP) level of 2.21 mg/dL (normal range, 0-0.3 mg/dL), aspartate aminotransferase (AST) of 2,689 IU/L (normal range, 0-40 IU/L), alanine aminotransferase (ALT) of 1,079 IU/L (normal range, 0-40 IU/L), total serum bilirubin of 1.6 mg/dL (normal range, 0-1.5 mg/dL), direct serum bilirubin of 1.4 mg/dL (normal range, 0-0.5 mg/dL), gamma-glutamyl transpeptidase of 69 IU/L (normal range, 11-49 mg/dL), serum creatinine kinase (CK) of 4,314 IU/dL (normal range, 24-204 IU/dL), and lactate dehydrogenase (LD) of 9,959 IU/L (normal range, 240-480 IU/L). Total serum protein and albumin was decreased to 5.5 g/dL (normal range, 6.0-8.2 g/dL) and 3.3 g/dL (normal range, 3.5-5.2 g/dL), respectively. Peripheral blood cell morphology revealed left-shifted maturation of granulocytes and moderate thrombocytopenia, while hemolysis was not observed. Coagulation studies revealed a prothrombin time (PT) of 18.5 seconds (normal range, 12.6-14.9 seconds), and 1.57 international normalized ratio (INR; normal range, 0.90-1.10 INR), activated partial thromboplastin time (aPTT) of 95.5 seconds (normal range, 29.1-41.9 seconds), fibrinogen of 99 mg/dL (normal range, 182-380 mg/dL), antithrombin III activity of 75% (normal range, 83-123%). Other laboratory exams including blood urea nitrogen, creatinine, electrolytes, ammonia, lactic acid, amylase, and lipase were in normal range.
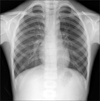
Chest radiography conducted on admission was normal without any lesions in the lung (Fig. 1). Computed tomography (CT) scans of the abdominal revealed mild hepatomegaly with periportal edema (Fig. 2A). Diffuse edematous change of the gallbladder and small amount of ascites was also found on CT images (Fig. 2B).
Serum antibody and polymerase chain reaction(PCR) tests to rule out other infections, including hepatitis A, hepatitis B, hepatitis C, cytomegalovirus, Epstein-Barr virus, herpes simplex virus, human herpes virus 6, varicella zoster virus, parvorvirus B19, toxoplasmosis and were all negative. Respiratory PCR tests of respiratory viruses including adenovirus, influenza, parainfluenza, respiratory syncytial virus, metapneumovirus, rhinovirus, coronavirus were also all negative. Ceruloplasmin level was 33.8 mg/dL (normal range, 20-60 mg/dL), and autoantibodies including anti-nuclear antibody, anti-smooth muscle antibody, and anti-mitochondrial antibody were all negative. Thyroid function tests were also normal. Serum antibodies to M. pneumoniae detected by enzyme-linked immunosorbent assay (ELISA) were 16.1 AU/mL for immunoglobulin (Ig) G, and 1.3 index value (ratio between the absorbance value of the test sample and that of the cut-off) for IgM. Serum levels for cardiac troponin I, CK-MB, and N-terminal pro-brain natriuretic peptide were all in normal range. Transthoracic echocardiogram findings were unremarkable. Serum isoenzyme electrophoresis revealed 100% of CK-MM, while CK-MB and CK-BB both constituted 0%.
The patient received empirical antibiotics of intravenous cefotaxime and clarithromycin, with daily supplements of fresh frozen plasma and cryoprecipitates. Fever and abdominal pain subsided on the third hospital day and laboratory exams showed gradual improvement. Although tachycardia was observed during the events of fever, blood pressure was continuously within normal range, and no oliguria was observed. Laboratory exams performed on the seventh hospital day revealed a hemoglobin of 11.8 g/dL, hematocrit 34.7%, WBC count of 7,710/mm3 with 27% neutrophils, 49% lymphocytes, 15% monocytes, and platelet count of 278,000/mm3. Chemistry exams revealed a CRP level of 0.32 mg/dL (normal range, 0-0.3 mg/dL), AST of 203 IU/L (normal range, 0-40 IU/L), ALT of 281 IU/L (normal range, 0-40 IU/L), total serum bilirubin of 0.7 mg/dL (normal range, 0-1.5 mg/dL), direct serum bilirubin of 0.3 mg/dL (normal range, 0-0.5 mg/dL), total serum protein of 6.5 g/dL (normal range, 6.0-8.2 g/dL), serum albumin of 3.8 g/dL (normal range, 3.5-5.2 g/dL), CK of 457 IU/dL (normal range, 24-204 IU/dL), and LD of 3,919 IU/L (normal range, 240-480 IU/L). Coagulation studies revealed a PT of 13.0 seconds (normal range, 12.6-14.9 seconds), and 1.04 INR (normal range, 0.90-1.10 INR), aPTT of 31.1 seconds (normal range, 29.1-41.9 seconds), fibrinogen of 93 mg/dL (normal range, 182-380 mg/dL). The patient was discharged on the eighth hospital day without any complaints nor any remaining signs and symptoms.
The patient was followed at the outpatient clinic two weeks after discharge. Laboratory exams showed normalization of levels that were above normal range prior to discharge, revealing a CRP of 0.03 mg/dL, AST of 33 IU/L, ALT of 24 IU/L, CK of 87 IU/L, LD of 412 IU/L. Serum antibodies to M. pneumoniae detected by ELISA were 79.6 AU/mL for IgG, and 2.6 index value for IgM, confirming the diagnosis of M. pneumoniae infection.
M. pneumoniae are primarily mucosal pathogens, which attach and enter the ciliated cells of the respiratory tract to produce proinflammatory cytokines causing acute cellular inflammatory reaction and resultant airway damage [4]. Hence, the consequence of M. pneumoniae infection is mainly pneumonia, known to account for up to 40% of cases of community-acquired pneumonia in children [4]. Although pneumonia is well known as the hallmark of M. pneumoniae infection, cases of extrapulmonary manifestations presenting in the absence of pneumonia have been rarely reported in both children and adults [23567]. The pathomechanism of how extrapulmonary manifestations are capable of occurring in the absence of pneumonia remains unknown. However it seems that extrapulmonary manifestations due to M. pneumoniae infection occur according to one of the following mechanisms; 1) a direct type in which local inflammatory cytokines plays an important role induced by M. pneumoniae at the site of inflammation, 2) an indirect type in which autoimmunity or the formation of immune complexes plays an important role in the absence of M. pneumoniae at the site of inflammation, and 3) a vascular occlusion type in which obstruction of blood flow plays an important role, induced either directly or indirectly by M. pneumoniae infection [78].
Elevated liver enzymes are frequently observed during M. pneumoniae infection in children. According to previous studies, 8-30% of children presenting with serologically confirmed M. pneumoniae infection had an evidence of liver involvement [910]. Based on the duration from the onset of fever and liver enzyme elevation, it seems that hepatic involvement of M. pneumoniae is bimodal [7]. While liver dysfunction is observed at the first hospital visit in early-onset hepatitis, it becomes evident later in the course, usually 7-10 days after the onset of fever, in late-onset hepatitis [7]. Narita et al. [3] reported that bacteremia with M. pneumoniae might be required for early-onset hepatitis, implying that early-onset hepatitis, which sometimes develops in the absence of pneumonia, may be related with a direct-type extrapulmonary manifestation [3567]. Meanwhile, late-onset hepatitis may be related with an indirect-type mechanism, in which cross-reactive antibodies induced by M. pneumoniae interact with sialo-oligosaccharides on hepatic cells [811].
In our case, body temperature elevation up to 37.6℃ and mild cough had been documented 10 days before admission, although fever over 38℃ developed just 4 days before admission. Thus, there is a possibility that primary infection with M. pneumonia may have started 10 days prior to admission. The self-limiting course of mild cough and body temperature elevation within a 2 days period may explain the mild course of pulmonary involvement and the absence of pneumonia in our case. Meanwhile, fever over 38℃ developed concurrently with abdominal pain in the right upper quadrant, 4 days after the resolution of the initial body temperature elevation. This clinical course of a 4 day symptom-free window period implies that the acute cholestatic hepatitis in our case may have been related with an indirect-type mechanism rather than a direct type mechanism.
The concurrent symptoms and signs of other extrapulmonary manifestations, such as erythematous maculopapular skin rash, rhabdomyolysis, and initial laboratory exams favoring disseminated intravascular coagulopathy (DIC) in our case further support the assumption that our case may have been related with an indirect-type mechanism rather than a direct type mechanism. Association between M. pneumonia infection and dermatologic manifestations such as erythematous maculopapular eruptions and erythema multiforme are well known [12], and the molecular mimicry between Mycoplasma P1-adhesin molecule and keratinocyte antigen leading to the generation of cross-reacting antibodies, immune-complex formation, and complement activation have been reported to attribute to the development of Mycoplasma induced rash and mucositis (MIRM) [13]. Rhabdomyolysis is often accompanied by multiple extrapulmonary manifestations in M. pneumoniae infection as in our case [714], although the exact underlying pathomechanism has not been revealed. Recently, it has been suggested that tumor necrosis factor-alpha may play a role in the pathogenesis of rhabdomyolysis associated with M. pneumoniae infection [15]. The development of DIC in M. pneumoniae infection is related with the vascular occlusion type mechanism in the presence of a systemic hypercoagulable state [7]. Abnormal immune regulation in M. pneumoniae infection has been suggested to activate complements or induce procoagulant mediators attributing to the development of DIC [161718]. Therefore, our case may have been related with an indirect-type plus a vascular occlusion type mechanism.
The main basis of treatment in cases of extrapulmonary manifestations associated with M. pneumoniae infection is antibiotics effective against the organism. Antibiotics such as macrolides should be administered to reduce the amount of M. pneumoniae infected cells in the respiratory tract, which leads to the reduction of excessive antigenic stimuli [8]. The emergence and increasing incidence of macrolide resistant M. pneumoniae strains, especially in East Asian countries, has aroused attention in the treatment of M. pneumoniae infection [1920]. A recent review suggested the introduction of other potentially effective antibiotics in cases of clinical deterioration of symptoms or signs despite treatment with first line macrolides, in which the choice of the replaced antibiotic should be decided according to their in vitro activity and potential adverse events [20]. Corticosteroids or immunoglobulins are suggested to be beneficial in severe cases, and anticoagulation therapy is considered highly promising for cases of vascular occlusion type manifestations [8]. In our case, early suspicion of M. pneumoniae infection based on the patient's history of mild cough, and the administration of clarithromycin could have played a crucial role in the early improvement of the disease course, despite the fact that respiratory involvement M. pneumoniae seemed to be mild in our case.
In conclusion, we report the first pediatric case in Korea of M. pneumoniae infection in a child presenting with acute cholestatic hepatitis in the absence of pneumonia. The clinical course and concurrent multiple extrapulmonary manifestations indicate that an indirect-type plus a vascular occlusion type mechanism may have played a role in the pathomechanism of extrapulmonary manifestations in our case. This case also emphasizes that M. pneumoniae infection should be considered as a possible cause of acute cholestatic hepatitis despite the absence of pneumonia, and that early treatment with proper antibiotics should be initiated from suspicion.
Figures and Tables
References
1. Waites KB. New concepts of Mycoplasma pneumoniae infections in children. Pediatr Pulmonol. 2003; 36:267–278.
2. Arav-Boger R, Assia A, Spirer Z, Bujanover Y, Reif S. Cholestatic hepatitis as a main manifestation of Mycoplasma pneumoniae infection. J Pediatr Gastroenterol Nutr. 1995; 21:459–460.

3. Narita M, Yamada S, Nakayama T, Sawada H, Nakajima M, Sageshima S. Two cases of lymphadenopathy with liver dysfunction due to Mycoplasma pneumoniae infection with mycoplasmal bacteraemia without pneumonia. J Infect. 2001; 42:154–156.

4. Atkinson TP, Waites KB. Mycoplasma pneumoniae infections in childhood. Pediatr Infect Dis J. 2014; 33:92–94.

5. Romero-Gómez M, Otero MA, Sánchez-Muñoz D, Ramírez-Arcos M, Larraona JL, Suárez García E, et al. Acute hepatitis due to Mycoplasma pneumoniae infection without lung involvement in adult patients. J Hepatol. 2006; 44:827–828.

6. Quioc JJ, Trabut JB, Drouhin F, Malbrunot C, Vallet-Pichard A, Pol S, et al. Acute cholestatic hepatitis revealing Mycoplasma pneumoniae infection without lung involvement in an adult patient. Eur J Gastroenterol Hepatol. 2009; 21:220–221.

7. Narita M. Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother. 2010; 16:162–169.

8. Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. 2016; 7:23.

9. Kim KW, Sung JJ, Tchah H, Ryoo E, Cho HK, Sun YH, et al. Hepatitis associated with Mycoplasma pneumoniae infection in Korean children: a prospective study. Korean J Pediatr. 2015; 58:211–217.

10. Grüllich C, Baumert TF, Blum HE. Acute Mycoplasma pneumoniae infection presenting as cholestatic hepatitis. J Clin Microbiol. 2003; 41:514–515.

11. Shin SR, Park SH, Kim JH, Ha JW, Kim YJ, Jung SW, et al. Clinical characteristics of patients with Mycoplasma pneumoniae-related acute hepatitis. Digestion. 2012; 86:302–308.

12. Terraneo L, Lava SA, Camozzi P, Zgraggen L, Simonetti GD, Bianchetti MG, et al. Unusual eruptions associated with Mycoplasma pneumoniae respiratory infections: review of the literature. Dermatology. 2015; 231:152–157.

13. Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of Mycoplasma pneumoniae: an update. Indian J Med Microbiol. 2016; 34:7–16.

14. Weng WC, Peng SS, Wang SB, Chou YT, Lee WT. Mycoplasma pneumoniae--associated transverse myelitis and rhabdomyolysis. Pediatr Neurol. 2009; 40:128–130.

15. Oishi T, Narita M, Ohya H, Yamanaka T, Aizawa Y, Matsuo M, et al. Rhabdomyolysis associated with antimicrobial drug-resistant Mycoplasma pneumoniae. Emerg Infect Dis. 2012; 18:849–851.
16. Chryssanthopoulos C, Eboriadou M, Monti K, Soubassi V, Sava K. Fatal disseminated intravascular coagulation caused by Mycoplasma pneumoniae. Pediatr Infect Dis J. 2001; 20:634–635.

17. Chiou CC, Liu YC, Lin HH, Hsieh KS. Mycoplasma pneumoniae infection complicated by lung abscess, pleural effusion, thrombocytopenia and disseminated intravascular coagulation. Pediatr Infect Dis J. 1997; 16:327–329.

18. Fumarola D. Intravascular coagulation and Mycoplasma pneumoniae infection. Pediatr Infect Dis J. 1997; 16:1012–1013.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download