Abstract
Esophageal candidiasis is commonly seen in immunocompromised patients; however, candida esophagitis induced stricture is a very rare complication. We report the first case of esophageal stricture secondary to candidiasis in a glycogen storage disease (GSD) 1b child. The patient was diagnosed with GSD type 1b by liver biopsy. No mutation was found in the G6PC gene, but SLC37A4 gene sequencing revealed a compound heterozygous mutation (p.R28H and p.W107X, which was a novel mutation). The patient's absolute neutrophil count was continuously under 1,000/µL when he was over 6 years of age. He was admitted frequently for recurrent fever and infection, and frequently received intravenous antibiotics, antifungal agents. He complained of persistent dysphagia beginning at age 7 years. Esophageal stricture and multiple whitish patches were observed by endoscopy and endoscopic biopsy revealed numerous fungal hyphae consistent with candida esophagitis. He received esophageal balloon dilatation four times, and his symptoms improved.
Candida esophagitis commonly occurs in immunocompromised patients, such as those with leukemia, other malignant neoplasm, diabetes mellitus, longterm steroid therapy, and extended use of broad-spectrum antibiotics, immunosuppressants, or chemotherapeutic agents [1].
However, candida esophagitis induced stricture is a very rare complication [123456]. It is well known that frequent infections can occur in glycogen storage disease (GSD) 1b patients, but thus far, candida esophagitis has not been reported in GSD 1b patients. This is a first case report of esophageal stricture due to candidiasis in a GSD 1b child.
A 2-month-old boy contracted pneumonia with atelectasis and showed progressive abdomen distension and puffy facial feature. He was referred to Seoul National University Children's Hospital when he was 5-month-old.
His height was 67.5 cm (50-75th percentile) and body weight was 8.5 kg (50-75th percentile). Physical examination showed hepatomegaly that occurred 3 cm below the costal margin. His hemoglobin was 9.6 (reference range, 13-17) g/dL, his white blood cell count was 8,400 (reference range, 4,000-10,000)/µL, and his platelet count was 591×103 (reference range, 130-400×103)/µL. Liver function tests showed mild elevation of serum aspartate transaminase 248 (reference range, 0-40) U/L, alanine transaminase 109 (reference range, 0-40) U/L. His fasting glucose level was 20 (reference range, 70-110) mg/dL. His liver sonograph showed a fatty liver with hepatomegaly suggestive of GSD. A liver biopsy showed cytoplasmic ballooning with deposits of periodic acid-Schif positive and diastase-digestible materials, glycogen nuclei, macrovesicular fat globules, and portal fibrosis. No mutation was found in the G6PC gene, but SLC37A4 gene sequencing revealed a compound heterozygous mutation (p.R28H and p.W107X, which was a novel mutation). He was diagnosed with GSD 1b, and given cornstarch.
The patient complained of dysphagia and stunted growth beginning at age 5 years. His symptom progressed and he was admitted for further evaluation at 7 years of age. Esophagogram showed diffuse stricture and multiple small diverticulum in the mid-esophagus (thoracic vertebrae 3 levels) (Fig. 1A) and multiple whitish patches were observed using endoscopy (Fig. 1B). With clinical suspicion of candida esophagitis, he received oral fluconazole for 20 days. His dysphagia was persistent and he received a second endoscopy. The whitish patches had almost disappeared, but the esophageal stricture was persistent. He received esophageal balloon dilatation (EBD) three times. After the dilatation his symptoms were relieved, but when he was 11 years old, he complained of recurrent dysphagia, vomiting and hypoglycemia. An endoscopic biopsy revealed numerous fungal hyphae consistent with candida esophagitis.
The patient's absolute neutrophil count was continuously under 1,000/µL when he was over 6 years of age. He was admitted frequently for recurrent esophageal candidiasis, neutropenic fever, skin infection and acute otitis media (AOM). He frequently received intravenous antibiotics, antifungal agents and granulocyte colony-stimulating factor (G-CSF). However, recurrent AOM caused tympanic membrane adhesion, hearing defects, and frequent pneumonia finally ending with bronchiectasis with emphysematous changes.
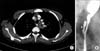
At the age of 21 years, he presented with progressive anterior lower chest pain. Chest computed tomography showed esophageal wall thickening and a left mediastinal abscess (Fig. 2A). In addition, the patient continued to experience bronchiectasis with mucous plugging. Esophagography showed a suspected esophageal fistulous track (Fig. 2B). However, we could not find a correlation between the esophagus and the mediastinal abscess. With intravenous antibiotics and fluconazole, the fever and dysphagia subsided.
When the patient was 22 year old, multiple variably sized nodules appeared in both hepatic lobes, with the largest one measured at 1.5 cm; these nodules appeared as continuously growing hepatic adenomas by magnetic resonance imaging.
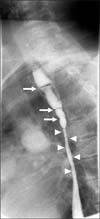
An endoscopy performed when the patient was 23 years old showed mid-esophageal stricture, and a 5 mm endoscope could not pass through the esophagus. Esophagography showed a diffuse narrowing of the mid-esophagus (arrowheads) and multiple concentric strictures in the upper esophagus (arrows) (Fig. 3).
He is currently 24 years old, his height is 173.1 cm (25-50th percentile), and his body weight is 59 kg (10-25th percentile). His most recent physical examination still showed hepatomegaly. The patient was suffering from severe progressive dysphagia. Multifocal tight strictures at the mid-esophagus (thoracic vertebrae 5 level) were observed on esophagography. He received his 4th EBD dilatation with a 10 mm×2 cm balloon catheter. After EBD, his dysphagia resolved, and he could eat solid food without difficulty, He is now planning to undergo liver transplantation because of multiple growing adenomas.
There are some reports of oral, perineal and inguinal skin candidiasis in GSD [7] but this is the first report of esophageal stricture due to candida esophagitis in a GSD type 1b patient. Our case is also the first report of the novel mutation c.320G>A (p.W107X) in the SLC37A4 gene.
Fungal esophagitis has been shown induce sloughing of the esophageal mucosa, resulting in stricture, but this condition is very rare [123456]. In a pediatric case, a 3-year-old leukemic patient received chemotherapy and radiotherapy. During treatment, she contracted a candida infection manifesting as pericarditis, oral candidiasis and genital candidiasis, and received antifungal agents. After treatment for the fungal infection, she had progressive dysphagia and a 6 cm esophageal stricture was observed [3]. Four pediatric acute lymphoblastic leukemia patients developed esophageal stricture, 2 of whom were confirmed as having candida esophagitis, whereas the other 2 patients had previous systemic candidiasis [4]. It was reported that the colonization of Candida albicans in the esophagus was not related to Barett's esophagus, paraesophageal hernia and esophageal diverticulum [8].
G6Pase plays a key role in the final step of glycogenolysis, which involves the hydrolysis of glucose-6-phosphate (G6P) in the endoplasmic reticulum. G6P translocase (G6PT), encoded by the SLC37A4 gene, is ubiquitously expressed, and transports G6P from the cytosol to the endoplasmic reticulum. Bi-allelic SLC37A4 mutations result in GSD 1b [910]. Our patient had two different mutations in the SLC37A4 gene, including the known mutation c.83G >A (p.R28H), and a novel mutation c.320G>A (p.W107X). The novel mutation was found at codon 107, mutating a tryptophan residue to a stop codon. In Korea, another novel mutation, c443C>T, was reported in one boy and led to the substitution of alanine by valine at codon 148 (A148V), causing GSD 1b [11].
Many studies have been conducted regarding the association between GSD 1b and neutropenia, and inactivation of G6PT induced neutrophil apoptosis, thus causing neutropenia and neutropenic dysfunction [1213]. GSD 1b patients experience recurrent infections and show inflammatory bowel disease (IBD) features in gastrointestinal tract that are difficult to distinguish from Crohn's disease [1214].
In neutropenia treatment, G-CSF can be used, not only to increase neutrophil count but also to improve neutrophil function and thus, reduce severe infection [15]. One case report, described peripheral stem cell transplantation as effective for 6.5-year-old GSD 1b patient suffering from neutropenic life-threatening complications, such as IBD, and recurrent infection. His IBD symptoms were relieved, his infection frequency and admission counts were decreased and G-CSF treatment was discontinued [14]. Live transplantation can be helpful for neutropenia and errors of metabolism in the liver, leading to an improved quality of life [161718]. Recently, gene therapy using an adenoviral vector expressing G6PT was studied in an animal model of neutropenia in GSD 1b patients [19].
Our patient had neutropenia for a long time and suffered from recurrent infection and complications including bronchiectasis, esophageal candidiasis and adhesive tympanic membrane, although he received the appropriate antibiotics and G-CSF. However, recurrent esophageal candidiasis induced mucosal glandular inflammation and resulted in stricture formation [2].
Our report is the first of esophageal stricture due to candidiasis in a GSD type 1b patient. This suggests that neutropenia management is important for GSD 1b patients to reduce significant infections and complications.
Figures and Tables
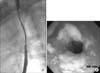
Fig. 1
Initial esophageal stricture and candidiasis. (A) There were diffuse strictures and multiple small diverticulum in the mid-esophagus (thoracic vertebrae 3 levels) on esophagography when he was 8 years old. (B) There were multiple whitish, dense, confluent plaques throughout the esophagus on esophagogastroduodenoscopy when he was 8 years old.
References
1. Ismail A, Abdulla S. Post-monilial extensive esophageal stricture. Pediatr Hematol Oncol. 1993; 10:111–113.

2. Kimura H, Kurachi M, Tsukioka Y, Minami M, Itou M, Fujii H, et al. Esophageal stricture secondary to candidiasis without underlying disease. J Gastroenterol. 1995; 30:508–511.

3. Dahl C, Fuursted K, Schrøder H. A paediatric case of Candida pericarditis and eosophagus stricture during treatment for acute lymphatic leukaemia. Acta Oncol. 2007; 46:859–861.

4. Kelly K, Storey L, O'Sullivan M, Butler K, McDermott M, Corbally M, et al. Esophageal strictures during treatment for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2010; 32:124–127.

5. Kim BW, Cho SH, Rha SE, Choi H, Choi KY, Cha SB, et al. Esophagomediastinal fistula and esophageal stricture as a complication of esophageal candidiasis: a case report. Gastrointest Endosc. 2000; 52:772–775.

6. Hyun JJ, Chun HJ, Keum B, Seo YS, Kim YS, Jeen YT, et al. Candida esophagitis complicated by esophageal stricture. Endoscopy. 2010; 42:Suppl 2. E180–E181.

7. Bartram CR, Przyrembel H, Wendel U, Bremer HJ, Schaub J, Haas JR. Glycogenosis type Ib complicated by severe granulocytopenia resembling inherited neutropenia. Eur J Pediatr. 1981; 137:81–84.

8. Andersen LI, Frederiksen HJ, Appleyard M. Prevalence of esophageal Candida colonization in a Danish population: special reference to esophageal symptoms, benign esophageal disorders, and pulmonary disease. J Infect Dis. 1992; 165:389–392.

9. Rake JP, Visser G, Labrune P, Leonard JV, Ullrich K, Smit GP. Glycogen storage disease type I: diagnosis, management, clinical course and outcome. Results of the European Study on Glycogen Storage Disease Type I (ESGSD I). Eur J Pediatr. 2002; 161:Suppl 1. S20–S34.

10. Banka S, Newman WG. A clinical and molecular review of ubiquitous glucose-6-phosphatase deficiency caused by G6PC3 mutations. Orphanet J Rare Dis. 2013; 8:84.

11. Han SH, Ki CS, Lee JE, Hong YJ, Son BK, Lee KH, et al. A novel mutation (A148V) in the glucose 6-phosphate translocase (SLC37A4) gene in a Korean patient with glycogen storage disease type 1b. J Korean Med Sci. 2005; 20:499–501.

12. Chou JY, Jun HS, Mansfield BC. Glycogen storage disease type I and G6Pase-β deficiency: etiology and therapy. Nat Rev Endocrinol. 2010; 6:676–688.

13. Kim SY, Jun HS, Mead PA, Mansfield BC, Chou JY. Neutrophil stress and apoptosis underlie myeloid dysfunction in glycogen storage disease type Ib. Blood. 2008; 111:5704–5711.

14. Pierre G, Chakupurakal G, McKiernan P, Hendriksz C, Lawson S, Chakrapani A. Bone marrow transplantation in glycogen storage disease type 1b. J Pediatr. 2008; 152:286–288.

15. Calderwood S, Kilpatrick L, Douglas SD, Freedman M, Smith-Whitley K, Rolland M, et al. Recombinant human granulocyte colony-stimulating factor therapy for patients with neutropenia and/or neutrophil dysfunction secondary to glycogen storage disease type 1b. Blood. 2001; 97:376–382.

16. Martinez-Olmos MA, López-Sanromán A, Martín-Vaquero P, Molina-Pérez E, Bárcena R, Vicente E, et al. Liver transplantation for type Ib glycogenosis with reversal of cyclic neutropenia. Clin Nutr. 2001; 20:375–377.

17. Adachi M, Shinkai M, Ohhama Y, Tachibana K, Kuratsuji T, Saji H, et al. Improved neutrophil function in a glycogen storage disease type 1b patient after liver transplantation. Eur J Pediatr. 2004; 163:202–206.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download